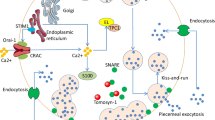
Abstract
Non-clonal mast cell activation disorder (syndrome) (NC-MCAD) and autoimmune/inflammatory syndrome induced by adjuvants (ASIA) are complex syndromic and spectral clinical entities which have contemporaneously garnered considerable attention and rightfully so. NC-MCAD presents with a predominantly allergic profile, and causative clinical variables may or may not be identified. Manifest NC-MCAD illnesses can be complex and involve several organ systems. Such diversity may be in part explained by the ubiquity and plasticity of the mast cell. ASIA, due to its broad definition, continues to expand as a clinical entity, especially with the greater recognition of what might constitute adjuvancy. Such diversity has the potential to overly stretch the boundaries for scientific investigation. For both NC-MCAD and ASIA, the inciting molecular mechanisms require elucidation. Future research would benefit from greater precision for clinical diagnosis and risk factor identification, and the latter would be potentially facilitated by the discovery of reliable laboratory markers. Some patients with ASIA may present with clinical features that may be identified as NC-MCAD. This review examines the potential spectral nexus of NC-MCAD and ASIA and considers the role of the mast cell in such a possible interface.
Similar content being viewed by others
References
Hamilton MJ. Nonclonal mast cell activation syndrome: a growing body of evidence. Immunol Allergy Clin N Am. 2018;38(3):469–81.
Cohen Tervaert JW. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): a new flame. Autoimmun Rev. 2018;17(12):1259–64.
Watad A, Bragazzi NL, Amital H, Shoenfeld Y. Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast. Isr Med Assoc J. 2019;21(8):517–9.
Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ. Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol. 2011;128(1):147–52.
Cardet JC, Castells MC, Hamilton MJ. Immunology and clinical manifestations of non-clonal mast cell activation syndrome. Curr Allergy Asthma Rep. 2013;13(1):10–8.
Watad A, Quaresma M, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome)–an update. Lupus. 2017;26(7):675–81.
Colaris MJL, de Boer M, van der Hulst RR, Tervaert JWC. Two hundred cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017;65(1):120–8.
Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806–18.
Munn R, Farrell K, Cimolai N. Acute encephalomyelitis: extending the neurologic spectrum of acute rheumatic fever? Neuropediatrics. 1992;23(4):196–8.
Shulman ST, Ayoub EM. Poststreptococcal reactive arthritis. Curr Opin Rheumatol. 2002;1(5):562–5.
Murphy TK, Goodman WK, Ayoub EM, Voeller KK. On defining Sydenham’s chorea: where do we draw the line? Biol Psychiatry. 2000;47(10):851–7.
Gamucci A, Uccella S, Sciarretta L, et al. PANDAS and PANS: clinical, neuropsychological, and biological characterization of a monocentric series of patients and proposal for a diagnostic protocol. J Child Adolesc Psychopharmacol. 2019. https://doi.org/10.1089/cap.2018.0087.
Cimolai N. The Canadian contribution to the science of verotoxigenic Escherichia coli and associated illnesses: the early years. Can J Microbiol. 2013;59(11):709–915.
Carter JE, Cimolai N. Hemolytic-uremic syndrome associated with acute Campylobacter upsaliensis gastroenteritis. Nephron. 1996;74(2):489.
Cimolai N. Are Clostridium difficile toxins nephrotoxic? Med Hypotheses. 2019;126:4–8.
Cimolai N, Anderson JD, Bhanji NMF, Chen L, Blair GK. Escherichia coli O157:H7 infection associated with perforated appendicitis and chronic diarrhea. Eur J Pediatr. 1990;149(4):259–60.
Cimolai N, Blair GK, Murphy JJ, Fraser GG. The impact of verotoxigenic Escherichia coli O157:H7 infection on a pediatric surgical service. Can J Surg. 1997;40(1):28–32.
Cimolai N, Carter JE, Morrison BJ, Anderson JD. Risk factors for the progression of Escherichia coli O157: H7 enteritis to hemolytic-uremic syndrome. J Pediatr. 1990;116(4):589–92.
Cimolai N, Morrison BJ, Carter JE. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome in childhood. Pediatrics. 1992;90(4):616–21.
Steere AC, Taylor E, McHugh GL, Logigian EL. The overdiagnosis of Lyme disease. JAMA. 1993;269(14):1812–6.
Banerjee S, Banerjee M, Cimolai N, Malleson P, Proctor E. Seroprevalence survey of borreliosis in children with chronic arthritis in British Columbia, Canada. J Rheumatol. 1992;19(10):1620–4.
Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect. 2019;25(10):1233–8.
Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. 2017;33(2):183–93.
Frazier TW, Youngstrom EA, Embacher R, et al. Demographic and clinical correlates of autism symptom domains and autism spectrum diagnosis. Autism. 2014;18(5):571–82.
Blaslov K, Naranda FS, Kruljac I, Renar IP. Treatment approach to type 2 diabetes: past, present and future. World J Diabetes. 2018;9(12):209–19.
Bloomgarden Z. Beyond HbA1c. J Diabetes. 2017;9(12):1052–3.
Heianza Y, Arase Y, Fujihara K, et al. Screening for pre-diabetes to predict future diabetes using various cut-off points for HbA1c and impaired fasting glucose: the Toranomon Hospital Health Management Center Study 4 (TOPICS 4). Diabet Med. 2012;29(9):e279–85.
Brooks-Worrell B, Palmer JP. Is diabetes mellitus a continuous spectrum? Clin Chem. 2011;57(2):158–61.
Inzucchi SE. Chapter 18. Classification and diagnosis of diabetes mellitus. In: Porte Jr D, Sherwin RS, Baron A, editors. Ellenberg and Rifkin’s diabetes mellitus. 6th ed. New York: McGraw Hill Professional; 2002.
Zimmer P, Cowie C, Ekoe J-M, Shaw J. Chapter 1. Classification of diabetes mellitus and other categories of glucose intolerance. In: RA DF, Ferrannini E, Keen H, Zimmet P, editors. International textbook of diabetes mellitus, vol. 1. 3rd ed: John Wiley & Sons, Ltd.; 2004.
McIntyre HD. Discovery, knowledge, and action-diabetes in pregnancy across the translational spectrum: the 2016 Norbert Freinkel Award Lecture. Diabetes Care. 2018;41(2):227–32.
Espinosa E, Valitutti S. New roles and controls of mast cells. Curr Opin Immunol. 2018;50:39–47.
González-de-Olano D, Álvarez-Twose I. Mast cells as key players in allergy and inflammation. J Investig Allergol Clin Immunol. 2018;28(6):365–78.
Brown MA. Studies of mast cells: adventures in serendipity. Front Immunol. 2018;9:520. https://doi.org/10.3389/fimmu.2018.00520.
Fieri M, Patel R, Celestin J. Mast cell activation syndrome: a review. Curr Allergy Asthma Rep. 2013;13(1):27–32.
Álvarez-Twose I, González de Olano D, Sánchez-Muňoz L, et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol. 2010;125(6):1269–78.
Maric I, Sun X. Advances in diagnosis of mastocytosis and hypereosinophilic syndrome. Semin Hematol. 2018;56(1):22–9.
Tefferi A, Shah S, Reichard KK, Hanson CA, Pardanani A. Smoldering mastocytosis: survival comparisons with indolent and aggressive mastocytosis. Am J Hematol. 2019;94(1):E1–2.
Shibao C, Arzubiaga C, Roberts LJ 2nd, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45(3):385–90.
Kassab D, Koterba A, Jiang, Akin C. Elevated tryptase levels in patients with mast cell activation syndromes without evidence of mastocytosis. J Allergy Clin Immunol. 2008;121(Suppl):S67.
Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126(6):1099–104.
Valent P, Akin C, Bonadonna P, et al. Mast cell activation syndrome: importance of consensus criteria and call for research. J Allergy Clin Immunol. 2018;142(3):1008–10.
Valent P, Akin C, Bonadonna P, et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125–33.
Schofield JR, Afrin LB. Recognition and management of medication excipient reactivity in patients with mast cell activation syndrome. Am J Med Sci. 2019;357(6):507–11.
Golden DBK. The many faces of mast cell disorders–a house of mirrors? J Allergy Clin Immunol Pract. 2019;7(4):1139–41.
Afrin LB, Molderings GJ. A concise, practical guide to diagnostic assessment for mast cell activation disease. World J Hematol. 2014;3(1):1–17.
Kabashima K, Nakashima C, Nonomura Y, et al. Biomarkers for evaluation of mast cell and basophil activation. Immunol Rev. 2018;282(1):11–20.
Weiler CR. MCAS: tools for diagnosis and differential diagnosis. J Allergy Clin Immunol Pract. 2020;8(2):498–506.
Valent P, Bonadonna P, Hartmann K, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180(1):44–51.
Shoenfeld Y, Agmon-Levin N. ‘ASIA’–autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8.
Sergott TJ, Limoli JP, Baldwin CM, Laub DR. Human adjuvant disease, possible autoimmune disease after silicone implantation: a review of the literature, case studies, and speculation for the future. Plast Reconstr Surg. 1986;78(1):104–11.
Ostermeyer Shoaib B, Patten BM. Human adjuvant disease: presentation as a multiple sclerosis-like syndrome. South Med J. 1996;89(2):179–88.
Bizjak M, Selmi C, Praprotnik S, et al. Silicone implants and lymphoma: the role of inflammation. J Autoimmun. 2015;65:64–73.
Powell DL. Cutaneous reactions to silicone. Dermatitis. 2005;16(2):97–8.
Kurihara S, Tani Y, Tateishi K, et al. Allergic eosinophilic dermatitis due to silicone rubber: a rare but troublesome complication of the Tenckoff catheter. Perit Dial Int. 1985;5(January–March):65–67.
Rubio A, Ponvert C, Goulet O, Scheinmann P, de Blic J. Allergic and nonallergic hypersensitivity reactions to silicone: a report of one case. Allergy. 2009;64(10):1554–61.
Hunsaker DH, Martin PJ. Allergic reaction to solid silicone implant in medial thyroplasty. Otolaryngol Head Neck Surg. 1995;113(6):782–4.
Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB Jr. Silicone allergy: a new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27(8):1078–82.
Oprea ML, Schnöring H, Sachweh JS, Ott H, Biertz J, Vazquez-Jimenez JF. Allergy to pacemaker silicone compounds: recognition and surgical management. Ann Thorac Surg. 2009;87(4):1275–7.
Stuck BA, Hecksteden K, Klimek L, Hörmann K. Type I hypersensitivity to a silicone tube after laryngectomy. HNO. 2004;52(3):255–7.
van der Walle HB, Klecak G, Geleick H, Bensink T. Sensitizing potential of 14 mono (meth) acrylates in the guinea pig. Contact Dermatitis. 1982;8(4):223–35.
Kanerva L, Estlander T, Jolanki R, Tarvainen K. Statistics on allergic patch test reactions caused by acrylate compounds, including data on ethyl methacrylate. Am J Contact Dermat. 1995;6(2):75–7.
Torres MC, Linares T, Hernandez MD. Acrylates induced rhinitis and contact dermatitis. Contact Dermatitis. 2005;53(2):114.
Goon AT-J, Bruze M, Zimerson E, Goh C-L, Koh DS-Q, Isaksson M. Screening for acrylate/methacrylate allergy in the baseline series: our experience in Sweden and Singapore. Contact Dermatitis. 2008;59(5):307–13.
Salles AG, Lotierzo PH, Gemperli R, et al. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008;121(5):1811–20.
Alijotas-Reig J, Garcia-Gimenez V, Llurba E, Vilardell-Tarrés M. Autoimmune/inflammatory syndrome (ASIA) induced by biomaterials injection other than silicone medical grade. Lupus. 2012;21(12):1326–33.
Aavall Severinsen M, Sommerlund M, Naeser K. Management of a cataract patient with known allergy to methyl methacrylate. JCRS Online Case Rep. 2014;2(3):68–70.
Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, Garcia-Gimenez V. Autoimmune/inflammatory syndrome induced by adjuvants–ASIA–related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res. 2018;66(1):120–40.
Scanzi F, Andreoli L, Martinelli M, et al. Are the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) and the undifferentiated connective tissue disease (UCTD) related to each other? A case-control study of environmental exposures. Immunol Res. 2017;65(1):150–6.
Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18(13):1217–25.
Ben-Hur N, Ballantyne DL, Rees TD, Seidman I. Local and systemic effects of dimethylpolysiloxane fluid in mice. Plast Reconstr Surg. 1967;39(4):423–6.
Solomon G. A clinical and laboratory profile of symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24(1 Suppl. 1):29–37.
Vasey FB, Zarabadi SA, Seleznick M, Ricca L. Where there’s smoke there’s fire: the silicone breast implant controversy continues to flicker: a new disease that needs to be defined. J Rheumatol. 2003;30(10):2092–4.
Watad A, Quaresma M, Bragazzi NL, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld’s syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clin Rheumatol. 2018;37(2):483–93.
Fryzek JP, Signorello LB, Hakelius L, et al. Self-reported symptoms among women after cosmetic breast implant and breast reduction surgery. Plast Reconstr Surg. 2001;107(1):206–13.
Cruz-Tapias P, Blank M, Anaya J-M, Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24(4):389–93.
Katzav A, Kivity S, Blank M, Shoenfeld Y, Chapman J. Adjuvant immunization induces high levels of pathogenic antiphospholipid antibodies in genetically prone mice: another facet of the ASIA syndrome. Lupus. 2010;21(2):210–8.
Mendes J Jr, Mendes Maykeh VA, Frascino LF, Zacchi FFS. Gluteal implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2010;144(3):610–3.
Kumagai Y, Abe C, Shiokawa Y. Scleroderma after cosmetic surgery: four cases of human adjuvant disease. Arthritis Rheum. 1979;22(5):532–7.
Kaiser W, Biesenbach G, Stuby U, Grafinger P, Zazgornik J. Human adjuvant disease: remission of silicone induced autoimmune disease after explantation of breast augmentation. Ann Rheum Dis. 1990;9:937–8.
Goren I, Segal G, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvant (ASIA) evolution after silicone implants–who is at risk? Clin Rheumatol. 2015;34(10):1661–6.
Travis WD, Balogh K, Abraham JL. Silicone granulomas: report of three cases and review of the literature. Hum Pathol. 1985;16(1):19–27.
Šmahel J. Histology of the capsules causing constrictive fibrosis around breast implants. Br J Plast Surg. 1977;30(4):324–9.
Smalley DI, Shanklin DR. T-cell-specific response to silicone gel. Plast Reconstr Surg. 1996;98(5):915–6.
Kalicharan D, Jongebloed WL, Van Der Veen G, Los LI, Worst JGF. Cell-ingrowth in a silicone plombe: interactions between biomaterial and scleral tissue after 8 years in situ: a SEM and TEM investigation. Doc Ophthalmol. 1991;78(3–4):307–15.
Heggers JP, Kossovsky N, Parsons RW, Robson MC, Pelley RP, Raine TJ. Biocompatibility of silicone implants. Ann Plast Surg. 1983;11(1):38–45.
Naim JO, Lanzafame RJ, van Oss CJ. The adjuvant effect of silicone-gel on antibody formation in rats. Immunol Investig. 2009;22(2):151–61.
Narini PP, Semple JL, Hay JB. Repeated exposure to silicone gel can induce delayed hypersensitivity. Plast Reconstr Surg. 1995;96(2):371–80.
Kossovsky N, Heggers JP, Robson MC. Experimental demonstration of the immunogenicity of silicone-protein complexes. J Biomed Mater Res. 1987;21(9):1125–33.
Hinderer UT, Escalona J. Dermal and subdermal tissue filling with fetal connective tissue and cartilage, collagen, and silicone: experimental study in the pig compared with clinical results–a new technique of dermis mini-autograft injections. Aesthet Plast Surg. 1990;14(4):239–48.
Lilla JA, Vistnes LM. Long-term study of reactions to various silicone breast implants in rabbits. Plast Reconstr Surg. 1976;57(5):637–49.
Andrews JM. Cellular behavior to injected silicone fluid: a preliminary report. Plast Reconstr Surg. 1966;38(6):581–3.
Nosanchuk JS. Injected dimethylpolysiloxane fluid: a study of antibody and histologic response. Plast Reconstr Surg. 1968;42(6):562–6.
Janowsky EC, Kupper LL, Hulka BS. Meta-analysis of the relation between silicone breast implants and the risk of connective-tissue disease. N Engl J Med. 2000;342(11):781–90.
Ameratunga R, Gillis D, Gold M, Linneberg A, Elwood JM. Evidence refuting the existence of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). J Allergy Clin Immunol Pract. 2017;5(6):1551–5.
Ameratunga R, Langguth D, Hawkes D. Perspective: scientific and ethical concerns pertaining to animal models of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). Autoimmun Res. 2018;17(5):435–9.
Groth AK, Graf R. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthet Plast Surg. 2019. https://doi.org/10.1007/s00266-019-01521-3.
Souto LRM. Invited discussion on: breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthet Plast Surg. 2019. https://doi.org/10.1007/s00266-019-01528-w.
McCulley JP. Biocompatibility of intraocular lenses. Eye Contact Lens. 2003;29(3):155–63.
Lloyd AW, Faragher RGA, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22(8):769–85.
Hall BJ, Jones LW, Dixon B. Silicone allergies and the eye: fact or fiction? Eye Contact Lens. 2014;40(1):51–7.
Stills HF Jr. Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005;46(3):280–93.
van Oss CJ, Singer JM, Gillman CF. The influence of particulate carriers and of mineral oil in adjuvants on the antibody response in rabbits to human gamma globulin. Immunol Commun. 1976;5(3):181–8.
Broderson JR. A retrospective review of lesions associated with the use of Freund’s adjuvant. Lab Anim Sci. 1989;39(5):400–5.
Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Freund’s complete adjuvant: an effective but disagreeable formula. Res Immunol. 1992;143(5):478–83.
Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70(6):849–60.
Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;18:184–202.
Rose NR. Autoimmunity, infection and adjuvants. Lupus. 2010;19(4):354–8.
Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40(3):193–200.
Murray PJ, Young RA, Daley GQ. Hematopoietic remodeling in interferon-γ-deficient mice infected with mycobacteria. Blood. 1998;91(8):2914–24.
Meltzer MS, Leonard EJ. Enhanced tumor growth in animals pretreated with complete Freund’s adjuvant. J Natl Cancer Inst. 1973;50(1):209–18.
Mizisin AP, Wiley CA, Hughes RAC, Powell HC. Peripheral nerve demyelination in rabbits after inoculation with Freund’s complete adjuvant alone or in combination with lipid haptens. J Neuroimmunol. 1987;16(3):381–95.
Bernini L, Manzini CU, Giuggioli D, Sebastiani M, Ferri C. Reactive arthritis induced by intravesical BCG therapy for bladder cancer: our clinical experience and systematic review of the literature. Autoimmun Rev. 2013;12(12):1150–9.
Mizutani H, Mizutani H. Immunologic responses in patients with Mycoplasma pneumoniae infections. Am Rev Respir Dis. 1983;127(2):175–9.
Cimolai N, Mah D, Thomas E, Middleton PJ. Rapid immunoblot method for diagnosis of acute Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1990;9(3):223–6.
Cimolai N. Chapter 3. Heterophil antibody. In: Cimolai N, editor. Serodiagnosis of the infectious diseases: Mycoplasma pneumoniae. Boston: Kluwer Academic Publishers; 1999.
Cimolai N, Cheong ACH. Anti-smooth muscle antibody in clinical human and experimental animal Mycoplasma pneumoniae infection. J Appl Microbiol. 1997;82(5):625–30.
Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37.
Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59.
Blank M, George J, Barak V, Tincani A, Koike T, Shoenfeld Y. Oral tolerance to low dose β2-glycoprotein I: immunomodulation of experimental antiphospholipid syndrome. J Immunol. 1998;161(10):5303–12.
Grootens J, Understedt JS, Nilsson G, Dahlin JS. Deciphering the differentiation trajectory from hematopoietic stem cells to mast cells. Blood Adv. 2018;2(17):2273–81.
Johnson-Weaver B, Choi HW, Abraham SN, Staats HF. Mast cell activators as novel immune regulators. Curr Opin Pharmacol. 2018;41:89–95.
Frossi B, Mion F, Sibilano R, Danelli L, Pucillo CEM. Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity? Immunol Rev. 2018;282(1):35–46.
Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–94.
Woolley DE, Tetlow LC. Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Arthritis Res. 2000;2(1):65–74.
Pastwińska J, Agier J, Dastych J, Brzezińska-Blaszczyk E. Mast cells as the strength of the inflammatory process. Pol J Pathol. 2017;68(3):187–96.
Bonnekoh H, Scheffel J, Kambe N, Krause K. The role of mast cells in autoinflammation. Immunol Rev. 2018;282(1):265–72.
Ribatti D, Tamma R, Ruggieri S, Annese T, Marzullo A, Crivellato E. Mast cells and primary systemic vasculitides. Microcirculation. 2018;25(8):e12498. https://doi.org/10.1111/micc.12498.
Conti P, Caraffa A, Mastrangelo F, et al. Critical role of inflammatory mast cell in fibrosis: potential therapeutic effect of IL-37. Cell Prolif. 2018;51(5):e12475. https://doi.org/10.1111/cpr.12475.
Bradding P, Pejler G. The controversial role of mast cells in fibrosis. Immunol Rev. 2018;282(1):198–231.
Falanga V, Soter NA, Altman RD, Kerdel FA. Elevated plasma histamine levels in systemic sclerosis (scleroderma). Arch Dermatol. 1990;126(3):336–8.
Qureshi AA, Friedman AJ. A review of the dermatologic symptoms of idiopathic mast cell activation syndrome. J Drugs Dermatol. 2019;18(2):162–8.
Skaper SD, Facci L, Zusso M, Giusti P. Neuroinflammation, mast cells, and glia: dangerous liaisons. Neuroscientist. 2017;23(5):478–98.
Forsythe P. Mast cells in neuroimmune interactions. Trends Neurosci. 2019;42(1):43–55.
Costela-Ruiz VJ, Illescas-Montes R, Pavón-Martinez R, Ruiz C, Melguizo-Rodriguez L. Role of mast cells in autoimmunity. Life Sci. 2018;209:52–6.
Aoun Sebaiti M, Abrivard M, Blanc-Durant P, et al. Macrophagic myofasciitis-associated dysfunctioning: an update of neuropsychological and neuroimaging features. Best Pract Res Clin Rheumatol. 2018;32(5):640–50.
Kohn A, Chang C. The relationship between hypermobile Ehlers-Danlos syndrome (hDES), postural orthostatic tachycardia syndrome (POTS), and mast cell activation syndrome (MCAS). Clin Rev Allergy Immunol. 2019. https://doi.org/10.1007/s12016-019-08755-8.
Turner SD, Inghirami G, Miranda RN, Kadin ME. Cell of origin and immunological events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Am J Pathol. 2020;190(1):2–10.
Martinez-Villareal AA, Asz-Sigall D, Gutiérrez-Mendoza D, et al. A case series and a review of the literature on foreign modelling agent reaction: an emerging problem. Int Wound J. 2017;14(3):546–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest.
Human and Animal Rights
The review does not invoke human or animal research de novo.
Informed Consent
Informed consents are not required for this review.
Disclaimer
There are no relationships with pharmaceutical enterprises.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Cimolai, N. Mast Cell Biology and Linkages for Non-clonal Mast Cell Activation and Autoimmune/Inflammatory Syndrome Induced by Adjuvants. SN Compr. Clin. Med. 2, 2310–2323 (2020). https://doi.org/10.1007/s42399-020-00494-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00494-8