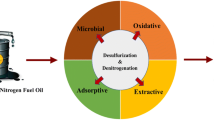
Abstract
The global demand for cleaner and more sustainable energy sources has stimulated extensive research into advanced technologies for sulfur removal from fossil fuels. Sulphur compounds in fuels contribute to air pollution, acid rain, and environmental degradation, underscoring the urgency of efficient desulfurisation methods. Deep eutectic solvents (DESs) have emerged as environmentally friendly candidates for sulphur removal from fuels. This review provides an overview of recent advancements in utilising DESs for desulfurisation processes, highlighting their sustainable and economically viable features. The manuscript begins with an introduction to the pressing need for clean fuels. It also emphasises the unique properties of DESs, such as low toxicity, biodegradability, and tunability, making them well-suited for sulphur extraction. A brief discussion on the classification and synthesis of DESs contextualises the review. Additionally, an overview of the effects of sulphur on the environment is presented. This review systematically categorises DESs used for desulfurisation into five distinct groups: DESs containing transition metal salts, sulfones, glycols, aromatic compounds, and organic acids. Each category is thoroughly discussed, examining their respective applications and effectiveness in desulfurisation processes. Towards the end, the review addresses current challenges and prospects in the field, including scaling up DES-based processes and enhancing efficiency through catalysts and synergistic approaches.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rapid industrialisation and urbanisation have exacerbated environmental pollution issues, leading to adverse effects on the ecosystem. Recently, internationally recognised fuel standards mandated that the sulfur content of fuels does not exceed 0.5wt% [1]. As a result, nations all over the world have passed pertinent laws and regulations to limit the sulfur content of fuels [2]. Harmful substances originating from automobiles, including metals, aromatic compounds, and refractory elements like sulfur and nitrogen have been linked to a range of detrimental consequences, including the formation of acid rain [3, 4]. Sulfur not only causes operational challenges for refining machinery and catalyst contamination but also gives rise to the release of sulfur oxide gases (SOx), thereby posing direct threats to both human health and the environment [5]. The SOx released during the combustion of sulfur-containing fuels have adverse repercussions, as they can be inhaled or combined with atmospheric moisture to form acid rain [6].
The process of eliminating sulfur from the fuel is commonly referred to as "fuel desulfurisation." Currently, one of the primary difficulties faced by the fuel manufacturing industry is the need to remove sulfur [7]. Sulfur is typically present in fuels in the form of compounds such as mercaptans, thiophenes (TH), and their derivatives [4]. These compounds are often the primary culprits behind the emission of SOx during the combustion process [8]. Almashjary and colleagues have indicated that desulfurisation can be achieved through various methods, including hydrodesulfurisation, extractive desulfurisation, adsorptive desulfurisation, biodesulfurisation, and oxidative desulfurisation [9].
Among all the methods mentioned above, hydrodesulfurisation is the most widely used technique for eliminating sulfur compounds from fuels. It is highly efficient in producing low-sulfur fuel on a large scale, but it does require a significant amount of energy which is a huge drawback [10, 11]. Another limitation of hydrodesulfurisation is its inability to remove organosulfur compounds, such as TH and their derivatives, due to the stearic hindrance effect [12, 13]. In contrast, extractive desulfurisation and oxidative desulfurisation have gained attention as effective alternatives to hydrodesulfurisation for sulfur removal in fuels [14, 15]. The benefits of extractive desulfurisation include high selectivity, mild reaction conditions, and ease of operation [16]. A very high extraction rate can be achieved after multiple extractions, and the extractant can be reused after the reverse extraction operation and there is no need for catalysts, which greatly reduces costs. The appropriate extractant is mixed with the system to be separated, and the stratified two phases are separated after constant temperature stirring and other operations [17]. In addition, in extractive desulfurisation, a wide range of solvents based on amines and ethers can be used. However, many of these solvents do not meet the necessary standards and lack essential properties such as ease of preparation, biodegradability, and non-toxicity, among others [18]. So, considering the limitations of traditional methods of removing sulfur from fuels, there is a pressing need for new environmentally friendly methods and materials that combine high efficiency with reduced environmental impact.
Deep Eutectic Solvents (DESs) have emerged as environmentally friendly solvents in the twenty-first century and have found application in the removal of sulfur compounds from fuels. DESs are a mixture of two or more compounds that form a stable system with a low melting point. They are usually prepared by combining a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), which have a strong interaction [19]. This interaction happens when the HBD interacts with quaternary ammonium salts through charge delocalisation. This leads to a decrease in the melting point and the formation of DESs. The melting point of the DESs is lower than the individual components and even lower than an ideal liquid mixture. This is because the DESs components have different types of interactions [20]. The most common formula for DESs is Cat+X−zY, where Cat+ is a cation of ammonium, sulfonium, or phosphonium, X− is a Lewis base halide anion, Y is Lewis or Brønsted acid, and z is the number of Y molecules interacting with the X− anion [21]. For instance, the following types of complexes are formed when choline chloride, ChCl (HOC2H4N(CH3)3+Cl− or Ch+Cl−), the most commonly used HBA, is linked to an HBD as shown below:
The complex anion is denoted by [HBD]Cl−, wherein the alcohol or amide group of the HBD forms a hydrogen bond with the Cl− and acidic protons. DESs present several advantages, including easy synthesis using readily accessible raw materials, minimal toxicity, excellent biocompatibility, and high degradability when compared to ionic liquids (ILs) [22].
This review presents recent scientific and technological developments in the field, responding to the growing importance of sulfur removal from fuel oil. The overview begins with a brief introduction to the topic. Following this, the effects of sulfur are highlighted. Subsequently, the types and preparatory methods for DESs are examined concisely. In exploring advancements in utilising DESs for desulfurisation processes in fossil fuels, the mini-review underscores the sustainable and economically viable features of DESs, presenting them as promising alternatives for sulfur removal. To provide a structured overview, this review categorises DESs used for desulfurisation into five groups: DESs containing transition metal salts, DESs containing sulfones, DESs containing glycols, DESs containing aromatic compounds, and DESs containing organic acids. The discussion then shifts to the regeneration of DESs used for the removal of sulfur from fuels in addition to challenges and perspectives in the removal of sulfur from fuel using DESs. The primary goal of this review is to inspire and guide researchers in academic and industrial communities toward developing efficient technologies for sulfur removal from fuel using DESs. Furthermore, using the search terms "sulfur removal from fuels by deep eutectic solvents" in a Scopus search on November 15th, 2024, I observed a remarkable surge in the amount of published literature during the previous eight years, totalling 99 documents (Fig. 1a). Scopus data obtained also indicated that tremendous work on the removal of sulfur using DESs was conducted mainly by researchers in Chemical Engineering, Chemistry, Energy and Environmental Science (Fig. 1b).
2 Overview of the Effects of Sulfur
Implications of fuel's sulfur content pose a major risk to the environment, wildlife, automobiles, infrastructure, and public health. The impact of sulfur on various factors is covered in this section. Diesel fuel's sulfur content determines how much sulfur is present, which is one of the main sources of particulate matter (PM) and PM emissions. According to previous research, 2.0% of the sulfur in diesel fuel turns into direct PM [23]. Because PM, particularly PM 2.5, is linked to lung cancer and cardiovascular mortality, it is detrimental to both human and animal health [24]. Chronic conditions such as respiratory infections, asthma, heart problems, chronic bronchitis, and stroke can result from prolonged exposure to PM 2.5 [25]. In addition, the harmful effects of oil in contaminated environments are primarily attributed to sulphur compounds. These compounds, known for their high hydrophobicity and low solubility in water, tend to strongly bond with organic matter, resulting in their accumulation in soil and sediments. This accumulation is associated with significant toxic effects on ecosystems, including the potential for mutation and cancer [26].
Flue gas from diesel engine combustion includes PM, SO2, and nitrogen oxides (NOx). The primary components of acid rain and smog are formed when these gases combine with water, oxygen, and other substances in the atmosphere. Acid rain—primarily sulfuric and nitric acids—causes harm to agricultural lands, destroys forests, pollutes lakes, and ruins houses and other property. Reduced biodiversity is one of the ecological effects of acid rain, which also upsets the natural balance by changing the chemical composition [5]. Sulfurous smog, sometimes referred to as London smog, is a severe air pollutant that irritates people's eyes, noses, and lungs and is brought on by excessive levels of SOx in the atmosphere [27].
Combustion engines are impacted by sulfur in addition to environmental issues. Internal combustion engines are corroded by sulfur oxyacids that are formed from combustion products. Moreover, sulfur strongly adsorbs onto catalysts, impeding the adsorption of other pollutants such as hydrocarbons, nitrogen oxide, and carbon monoxide, which reduces the effectiveness of air pollution control systems. Additionally, chemicals containing sulfur deactivate catalysts utilised in the upgrading of hydrocarbons [28]. Sulfuric acid from acid rain can also damage statues, buildings, and other man-made objects by wearing down metal and hastening the deterioration of paint and stone. The exteriors of buildings and other architectural structures, including monuments, are also susceptible to the damaging effects of acid rain. Acid rain damage can be expensive, requiring materials to be restored or, in extreme situations, rebuilt, which raises maintenance expenses [29, 30].
When significant amounts of sulfur-containing compounds are released into the atmosphere, it negatively affects the economy of a nation and affects a wide area. Reports of significant economic losses as a result of acid rain exist. Eliminating sulfur from diesel fuel has the potential to yield substantial economic benefits, particularly if the expenses associated with desulfurisation technology decline [5].
3 Categories and Preparation Methods of DESs
Based on the use of complexing agents, such as organic salts, non-ionic metal halides, and their hydrates, to form a eutectic mixture, DESs are classified into five main types as illustrated in Fig. 2 [21]. Quaternary ammonium salts of metal chloride and chloroaluminate/imidazolium make up type I eutectic mixtures. Type I eutectics can be formed experimentally from a mixture of organic salts and different metal chlorides, such as CuCl, CuCl2, FeCl2, SnCl2, SnCl4, CdCl2, LiCl, and ZnCl2 [31]. Metal halide hydrates and quaternary ammonium salts make up type II eutectic mixtures [32]. Moreover, quaternary ammonium, sulfonium, and phosphonium salts as HBAs and carboxylic acids, amines, alcohols, and amides as HBDs make up type III eutectic mixtures. Because of their ability to solvate and liquefy a wide variety of transition metal salts and their oxides, type III eutectics have attracted a lot of attention and research [33]. Due to their numerous significant applications, such as extraction, liquid–gas separation, glycerol removal from biodiesel, catalyst reactions, nanoparticle synthesis, and electrodeposition of metals [34,35,36]. Types III DESs have advantages over other types. HBDs and metal halides make up type IV eutectic mixtures. Since inorganic cations have high charge densities, they typically cannot form eutectic mixtures [37]. Furthermore, thymol-menthol DES is an example of Type V, a novel kind of eutectic mixture that solely contains nonionic molecules of HBAs and HBDs [38]. DESs and ILs are similar in terms of their physical characteristics, but their chemical structures are very different. Because of the significance of the non-ionic type of DESs (Type V), which can significantly expand their properties and potential uses, both theoretical and experimental researchers are now very interested in studying this area. The components' adaptability may present numerous options for synthesising DESs with a great deal of configurable functionality. Figure 2 illustrates the major classes of DESs available in the literature.
Safe and affordable components are used in the preparation of DESs. Primarily, there are four main methods for DES synthesis. The most widely utilised is the thermal method, which involves directly mixing the components of HBA and HBD and thereafter heating the composition to specific temperatures, depending on their respective melting points, in a closed vessel under atmospheric pressure [22, 39]. For example, the first DES was prepared in a closed vessel at 80 °C using ChCl as HBA and urea (U) as HBD in a 1:2 molar ratio [40]. A different way of preparing DES is to combine HBD and HBA aqueous solutions in a specific ratio, freeze-dry these aqueous solutions at a lower temperature (i.e. between − 196 and 20 °C), and then obtain a clear, viscous liquid [41]. The third method known as vacuum evaporation, involves dissolving both HBA and HBD in water and vaporising the water in a rotatory evaporator at 50 °C to prepare DESs. Once obtained, the liquid formed (DES) is then kept in a desiccator containing silica gel until a steady weight is achieved [33, 42]. The last method is grinding, which involves combining the HBA and HBD components of DES in a mortar and grinding them with a pestle at room temperature and atmospheric pressure [43, 44]. For instance, in a mortar and pestle at room temperature, ChCl and chloroacetic acid or trichloroacetic acid were ground until a homogenous liquid was formed [44]. It is possible to prevent the formation of ester impurities between carboxylic acid and ChCl by using the grinding and stirring methods at room temperature.
The majority of DESs are highly hygroscopic and capable of absorbing water from the atmosphere, this behaviour is worthy of note. The physical characteristics of DESs would be impacted by the small amount of water that these hydrophilic compounds absorb from the air. Thus, in all preparation techniques, it is crucial to prepare a DES in dry conditions and to dry it afterwards. DES synthesis produces no waste and could be affordable for large-scale production because it does not require complex procedures, solvents, or byproducts. In a chemical process, atom economy refers to minimizing byproducts or waste material and optimising the incorporation of starting material into the final product [45]. Since every initial component is used entirely in the finished product, the DES's final product has 100% atom economy and avoids the need for a purity assessment [46].
4 Application of DESs in Desulfurisation of Fuel Oils
4.1 DESs Containing Transition Metal Salts
A few authors have employed DESs containing transition metal salts for the removal of sulfur from fuel successfully. For instance, Li et al. investigated the extraction capabilities of DESs composed of HBA, HBD, and transition metal salts for dibenzothiophene (DBT) removal from n-octane [47]. In their study, the authors explored various transition metal-based salts. They found that DES composed of tetrabutylammonium chloride (TBAC), polyethylene glycol (PEG) and FeCl3 (TBAC/PEG/FeCl3) exhibited the best removal ability, reaching 81.2% compared to other metal-ion DESs with lower efficiencies. Through the optimisation process (TBAC/PEG/FeCl3 at a mole ratio of 4:1:0.05), the authors were able to further improve the performance of this DES to 89.5% removal efficiency. The primary factors contributing to the efficient extraction of sulfur in this case were hydrogen bonding and coordination effects. TBAC/PEG/FeCl3 DES possessed a relatively high electron density owing to the availability of hydroxyl and ethoxy chains in the polymer (PEG), as well as the central metal ion. Furthermore, the external electron cloud distribution of the metal ion enhanced its interaction with DBT in the DES, thus explaining the superior extraction capacity of Fe compared to other metals. Notably, this extractant can be categorized as a chromogenic agent, as it undergoes a change in colour after the extraction process. However, it is pertinent to know that the regeneration of this DES is a complex process, and the use of ether-based solvents for recycling is not advisable, particularly in the context of environmentally friendly recycling, as ether solvents-based are highly flammable and could pose a fire risk during the recycling process [48].
Similarly, Jiang et al. conducted a study in which they prepared DESs based on ChCl for the removal of DBT from n-octane [49]. Their motivation stemmed from ChCl's non-toxic nature, and they manipulated the HBA structures by varying the alkyl chain length (butyl, octyl, dodecyl, and benzyl) as well as the nature of the alkyl chain. Among the DESs they investigated, the one composed of C12DMEAC (dodecyldimethylethanolamine) and FeCl3 demonstrated the highest efficiency in desulfurisation. On the other hand, the DES containing a benzyl-substituted HBA showed the lowest extraction efficiency. This suggests that DESs containing longer aliphatic compounds are more efficient in eliminating sulfur in comparison to those with shorter alkyl chain lengths. The authors concluded that the dominant interaction in these DESs is CH-π interaction, which is more pronounced than the commonly observed π-π interaction in most aromatic-based DESs. Additionally, the coordination effect of Fe with sulfur species contributes significantly to the efficiency of sulfur removal.
Furthermore, from n-octane and kerosene, Mao et al. examined the desulfurisation potential of Brønsted-Lewis acidic metal-based DES of phenylpropanoic acid (PPA) and ZnCl2 [50]. The DES serves as the extractant as well as a catalyst and the oxidant used was H2O2. After optimisation, the kerosene's desulfurisation efficiency was observed to be 76.2%. Moreover, after a single extraction step, the removal efficiency of ChCl/SnCl2 DES was 95.2, 94.5 and 92.1 for DBT, 3-methylthiophene (3-MTH), and TH, respectively from n-octane using 1,5-diphenylcarbazide as complexing agents [51]. The authors observed the extraction mechanism to be π-π interaction and π-complexation of Sn2+ with electron pair of sulphur of the analytes as revealed by the FT-IR technique like the previous studies.
4.2 DESs Containing Sulfones
Sulfones, which are a type of HBDs, can be combined with HBAs to create sulfone-based DESs. A sulfone is a compound featuring a sulfonyl group attached to two adjacent carbon atoms. Although very less studies have applied sulfone-based DESs in desulfurisation processes, more research is warranted to harness their potential in this context. In a study conducted by Hadj-Kali and colleagues, the removal of TH was examined using DESs derived from sulfolane (Sul), triethylene glycol (TEG), and ethylene glycol (EG) [52]. For the preparation of DESs, tetrabutylammonium bromide (TBAB) and methyltriphenylphosphonium (MTPPB) were utilised as HBAs. A molar ratio of 1:7 was required to form TBAB/Sul while mixing MTPPB led to a solid mixture. The DES composed of TBAB/Sul demonstrated a notable solute concentration distribution in the raffinate phase when compared to glycol-based DESs as depicted in Fig. 3. This behaviour indicates a high selectivity for TH extraction into the DES phase. Additionally, 1H NMR characterisation confirmed the absence of sulfolane in the oil layer, confirming less risk of cross-contamination of DES with n-heptane. After five extraction cycles, up to 98% of the targeted compound was successfully extracted into the TBAB/Sul DES phase.
Comparison of extraction efficiency of sulfone-based DES with glycol-based DESs for the removal of TH. The data were extracted from the available literature [52]
Lima and colleagues observed a similar trend mentioned earlier regarding the ability of sulfone-based DESs to extract sulfur [53]. They explored the impact of various HBAs such as TBAB, TBAC, and tetrabutylphosphonium bromide (TBPB) removal of Th from n-heptane. Their findings revealed that the order of Th removal from n-heptane follows the order TBAC/Sul > TBAB/Sul > TBPB/Sul at a 1:4 molar ratio. This indicates that ammonium-based HBAs, specifically TBAC and TBAB, exhibit greater effectiveness compared to the phosphonium-based HBA (TBPB). Interestingly, the phosphonium cation was found to favour the extraction of DBT, implying that the selectivity of sulfur removal depends on the nature of the HBA. In a single extraction step, more than 80.0% of the targeted compounds were successfully removed.
4.3 DESs Containing Glycols
DESs composed of glycols have also been used for the removal of sulfur-containing compounds from fuels. For instance, various DESs were prepared by mixing ChCl, tetramethyl ammonium chloride (TMAC) and TBAC HBAs with PEG, Gly, EG, and TEG HBDs for 300 min at 367.15 K for the removal of BT [54]. Under optimal conditions, about 82.8% BT was removed from n-octane using DES composed of TBAC/PEG at a 1:2 molar ratio within a single extraction step. After five extraction cycles, up to 99.48% BT was successfully removed. Similarly, two different studies by Lima et al. confirmed the high efficiency of TBAC/PEG DES in removing sulfur-containing compounds [53, 54]. To ascertain the effect of PEG molecular weight on desulfurisation, Lima et al. prepared DESs constituted by PEGs (200, 300, 400, and 600 gmol−1) and TBAC for the removal of TH and DBT from n-heptane [54]. The efficiency of DESs regarding desulfurisation was found to increase gradually with increasing PEG molecular weight. The desulfurisation of the targeted compounds was found to follow the order TBAC/PEG 200 < TBAC/PEG 300 < TBAC/PEG 400 ≈ TBAC/PEG 600 as demonstrated in Fig. 4.
Effect of average molecular weight of PEG on the removal of TH and DBT from n-heptane. The data were obtained from the available literature [54]
Shu and Sun conducted a study where they used TBAC/EG and TBAC/GLY DESs to eliminate 2-methylthiophene (2-MTH), and benzothiophene (BT) from gasoline [55]. The authors achieved a successful removal rate of approximately 99.3% for 2-MTH and 99.5% for BT using TBAC/EG and TBAC/Gly DESs, respectively, after five extraction cycles. It seems that the extraction of sulfur compounds is more effective when using HBD with longer alkyl chain lengths. The wrapping of the HBD around the chloride anion enhances the packing structure and ultimately provides enough active hydrogen atoms for efficient sulfur removal [56].
Recently, a novel DES system, composed of ChCl, benzene sulfonic acid (BSA), and EG, was developed for oxidative desulfurisation of DBT, 4,6-DMDBT and BT from n-octane [57]. Ternary DESs, offering advantages such as volatility and high activity, outperformed organic acid-based binary DESs. Optimal conditions, including DES/oil of 1:5, oxidant to the sulfur molar ratio (O/S) of 5 at 25 °C, resulted in 100% removal efficiency for DBT, 4,6-DMDBT, and BT within 2 h. In addition, ZnCl2/boric acid (BA)/PEG exhibited 98.7%, 95.3% and 81.6% removal ability, respectively, for DBT, 4-methyldibenzothiophene (4-MDBT), and 4,6-DMDBT under optimised conditions (T = 60 °C, O/S = 5, t = 3 h) [58]. The primary factor preventing the removal of sulfur compounds was their internal steric hindrance. Nevertheless, the synergistic effect of ZnCl2 and BA led to a stronger hydrogen bonding which boosted the removal process.
4.4 DESs Containing Aromatic Compounds
Researchers have explored the use of DESs containing aromatic compounds in extractive desulfurisation. For instance, Tang et al. investigated ternary DESs made up of chlorinated paraffins-52, AlCl3, and aromatic derivatives like toluene, p-xylene, o-xylene, ethylbenzene, chlorobenzene, and benzene for desulfurisation purposes [59]. Among these DESs, those composed of paraffins-52/AlCl3/toluene were found to be the most effective in removing three thiophenic sulfur compounds, namely 3-MTH, BT, DBT, as well as actual oil. The order of effectiveness was observed to be as follows: DBT < BT < 3-MTH for the three sulfur compounds studied as demonstrated in Fig. 5. This order follows the electron density density of the sulfur compounds investigated. Furthermore, the order of efficiency of all the DESs examined for the targeted compounds follows this sequence: toluene > p-xylene > o-xylene > ethylbenzene > benzene > chlorobenzene. The π-cloud of the benzene ring was suggested to be responsible for the desulfurisation of the target compounds. However, it is worth noting that the stability of an aromatic compound is often influenced by the inductive effect of the substituent group. Alkyl groups, being electron-donating, can enhance the aromatic stability of a molecule by directing electron density toward the π-π system. Consequently, TH can be easily extracted into more stable aromatic compounds through π-π interactions, as supported by this rationale. Li et al. also observed a similar interaction in their study, where dicationic ILs exhibited greater π-π associations with aromatic sulfides as opposed to mono-cationic IL [60].
The efficiency of chlorinated paraffins-52/AlCl3/toluene DES for the extraction of 3-MTH, BT, and DBT from n-heptane. The data were extracted from the available literature [59]
Furthermore, Zhao et al. prepared DESs by combining aromatic acid derivatives like benzoic acid (BA), o-hydroxybenzoic acid (OHBA), and o-methylbenzoic acid (OMBA) as HBAs with amine-based HBDs such as triethylamine (TEA), tributylamine (TBA), and trihexylamine (THA) [61]. These DESs were then used for the removal of sulfur-containing compounds like TH, BT, and DBT. It was noted that the performance of desulfurisation was enhanced as the chain length of the HBA decreased. DESs containing TEA as the HBD exhibited the highest extraction efficiency. Additionally, it was observed that DESs composed of OHBA were more effective at removing sulfur in comparison to BA-based DESs. The improved removal efficiency was ascribed to the availability of the electron-donating group adjacent to the carboxyl group of benzoic acid, which formed a p-π system between the OH group and the aromatic ring. This, in turn, increased the π-π interaction between the DES and the targeted analytes, resulting in improved extraction efficiency.
Also, Makoś and Boczkaj studied phenol-based DESs for the separation of TH, BT, and DBT from n-heptane [62]. The hydrophobic properties of phenol were identified as the main driving force behind the separation capabilities of these phenol-based DESs. These DESs displayed an impressive extraction capacity of up to 99.2%, especially when employing ChCl/Phenol DES, in contrast to DESs containing substituted phenol. Additionally, DESs incorporating unsubstituted phenol, which is less hydrophobic, were found to be a preferable choice since they facilitated the direct transfer of sulfur species into the extractant-rich phase. Nonetheless, hydrophilic phenolic DESs are well-suited for oxidative extractive desulfurisation, as they are adept at capturing oxidized sulfur due to their strong affinity for DES.
Recently, aromatic-based DES comprising phenol and formic acid (FA) has been successfully employed to remove BT, 4,6-DMDBT and DBT from n-octane [63]. The authors successfully achieved 66.3%, 99.1% and 99.8% removal for 4,6-DMDBT and DBT at 300C and O/S of 2. Stearic hindrance from methyl groups and electron density on the sulfur atom influenced the reaction activity, leading to a decrease in the removal efficiency of the analytes in the order of DBT > 4,6-DMDBT > BT [64]. When DES (phenol/FA) under the same conditions was employed to remove sulfur from diesel, 98.3% removal efficiency was actualised. Also, L-proline (L-Pro)/benzenesulfonic acid (BSA) DES was recently utilised to remove BT 4,6-DMDBT and DBT from n-octane [65]. The removal efficiencies of BT 4, 6-DMDBT and DBT were 99.5%, 97.2% and 100% were obtained by the authors at 70 °C and O/S of 5, respectively. Out of the three analytes, the interaction energy between DES and BT using density functional was found to be the strongest at − 63.36 kJ/mol. This could be the primary cause of BT's effective removal.
4.5 DESs Containing Organic Acids
DESs containing organic acids have been the subject of extensive research for their effectiveness in the extractive removal of sulfur from fuel. Jiang et al. investigated a DES composed of 1-methylimidazole (MIM) and diethanolamine (DEA) as HBAs, along with propionic acid (PA) and nitric acid (NA) as HBDs [66]. The study demonstrated that a high sulfur partition coefficient (KN) could be achieved when using MIM/PA DES (KN = 2.31), in contrast to the 0.21 KN obtained for MIM/NA DES. After four extraction cycles, a removal efficiency of 97.6% was realised at atmospheric conditions. Desulfurisation appears to favour propionic acid (PA) due to its structure, which readily provides hydrogen bonding sites during the extraction process. However, in the case of nitric acid, the presence of acid ions and their conjugate base restricts the interaction of sulfur with the DES components. This restriction results in the lower KN observed when NA is employed as an HBD.
DESs made up of TBAB and various organic acids, comprising FA, acetic acid (AA), propionic acid (PA), oxalic acid (OA), malonic acid (MA), and adipic acid (AdA), were developed by Li et al. [67]. These DESs were employed for the extractive desulfurisation of BT, TH and DBT from n-octane in addition to unknown sulfur from diesel and fluid catalytic cracking (FCC) gasoline. After three extraction steps, TBAB/FA DES was found to be capable of removing 98.3% of BT, outperforming longer-chain organic acids with lower removal percentages. Moreover, these DESs achieved the removal of up to 83.6% and 70.2% removal of unknown sulfur-containing compounds from FCC gasoline and diesel, respectively, under similar conditions. The extraction process begins with the formation of intermolecular forces between the active hydrogen and the thiophenic species through hydrogen bonding. This interaction leads to the destruction of the DES, leaving behind the sulfur-containing compounds in the extractant phase.
Additionally, Hao et al. utilised DESs composed of L-proline (L-Pro) as the Hydrogen Bond Acceptor (HBA), along with OA, MA, gallic acid (GA), and p-toluenesulfonic acid (p-TsOH) as HBDs for the desulfurisation of DBT [68]. It was found that L-Pro/p-TsOH DES exhibited the best performance and could remove approximately 99.0% of DBT within 120 min at 333.15 K. Other DESs resulted in very low extraction efficiencies. The order of efficiency of DESs for the extraction of DBT is depicted in Fig. 6. The authors observed that the aromatic ring of DBT was partially destroyed in the studied systems. Furthermore, the HBD component of the DES was found to oxidize to peroxysulfonic acid, which enhanced the catalytic oxidation of DBT into its oxidized form.
The efficiency of various organic acid-based-DESs to extract DBT. The data were obtained from the available literature [68]
Recently, with a low O/S of 2, the desulfurisation efficiencies of natural DES based on γ-valerolactone (γ-Val):FA successfully reached 100% DBT in 100 min at 25 °C from n-octane while saving energy [69]. In addition, 100% sulfur removal of BT, 4,6-DMDBT, and DBT could be accomplished in 4 min, 6 min, and 10 min, respectively, when the temperature was raised to 70 °C and the O/S ratio was raised to 5. Similarly, in 2023, Guan et al. screened some multi-catalytic active site DESs through experiments and density functional theory for the sole aim of finding the best DES capable of removing DBT, 4,6-DMDBT, and BT from n-0ctane [15]. After optimisation at 25 °C, 2.5 h, and O/S 2.5, 5-sulfosalicylic acid/FA (SSA/FA) DES respectively led to excellent removal efficiencies of sulfur compounds in the order of DBT > 4,6-DMDBT > BT, as presented in Fig. 7. The electron density of sulfur atoms in BT, DBT, and 4,6-DMDBT, is reported as 5.739, 5.785, and 5.760, respectively [70]. Higher electron density generally correlates with easier sulfur oxidation. Despite 4,6-DMDBT having the highest electron density, DBT exhibits higher desulfurisation capacity. The marginal impact of electron density can be attributed to a negligible difference of 0.002 between DBT and 4,6-DMDBT. The presence of two methyl groups in 4,6-DMDBT, creating significant steric hindrance, likely contributes to its lower desulfurisation efficiency compared to DBT. The details and performances of DESs used desulfurisation of different fuels are presented in Table 1 and the chemical structures of all sulfur compounds removed from fuel using DESs are shown in Fig. 8.
The efficiencies of SSA/FA DES on the removal of various sulfur-containing compounds. The data were obtained from the available literature [15]
5 Regeneration/Recycling of DESs
Regeneration after extraction and subsequent solvent recycling is an important solvent selection criterion. Because DESs are reusable and considered "green solvents," they will be less expensive and will not waste resources. Several techniques, including anti-solvent addition, membrane filtration, crystallisation, liquid–liquid extraction, and supercritical fluid extraction, have been proposed for the regeneration of a DES following its use in various processes [71]. The type of DES and the chemical makeup of the sulfur compounds that need to be eliminated have a significant impact on each method's efficiency [72]. When employing DESs for oxidative desulfurisation, anti-solvent addition is used to carry out the regeneration process; in the majority of studies, this anti-solvent is water.
According to published research, hydrophilic DESs can be restored by washing them with water [73]. Dual Bronsted acidic DESs (3-sulfopropyl)triethylammoniumchloride/OA showed 97.70% sulfur removal efficiency at 50 °C [74]. Decantation was used to collect the fuel's upper phase following the reaction. The system was supplemented with deionized water. After removing the white solid on the bottom, a clear solution was obtained. After the water evaporated, the DESs were regenerated at lower pressure. Sulfur elimination efficacy reached 93.40% in fifth-time recycling under comparable conditions. For regeneration, rotary evaporation can be used to remove water from the DESs and separate the upper oil phase [75]. In this case, three extractions of the DESs were performed using an equivalent volume of carbon tetrachloride. Under ideal circumstances, new H2O2 and recovered DESs are added to the model oil. However, after five cycles, the removal efficiency of DBT dropped from 98.65% to 97.16%. This can be explained by the fact that there was little DES loss during recovery and that the recovered DESs still contained residual sulfide oxidation products. In addition, Guan et al. found that SSA/FA DES exhibited excellent recyclability [15]. The upper oil phase separation and direct reuse of used DES without regeneration resulted in consistent high sulfur removal (96.2% for DBT) over four cycles.
Most recently, the reusability of γ-Val/FA DES was explored by extracting the model fuel through decantation after each desulfurization experiment [69]. Subsequently, another 10 mL of model fuel was added for subsequent oxidative desulfurisation processes. The recycling performance of γ-Val/FA DES demonstrated a consistent desulfurisation efficiency during the removal of DBT (Fig. 9). Without regeneration, each cycle only incurred a roughly 3% loss in DBT removal efficiency. Even after four reuses, γ-Val/4FA maintained a substantial 90.04% desulfurization efficiency for DBT. Aside from its effective DBT removal capability, γ-Val/4FA also offers the advantage of avoiding the energy costs associated with the regeneration process.
The performance of the regenerated γ-Val/FA DES during the removal of DBT after four cycles. The data were obtained from the available literature [69]
Ternary DESs ChCl/BA/PEG (1:1.5:1) demonstrated a high desulfurisation efficiency of 99.2% within 2 h at 60 °C with an O/S ratio of 6. After the reaction, the DES easily separated from the oil phase due to their immiscibility. FT-IR results of regenerated DES showed similar absorption peaks as the fresh ones, indicating stability in the DES structure during the reaction. However, sulfur elimination decreased to 80.8% in the presence of used DESs without regeneration, likely due to the accumulation of oxidative products as previously mentioned. After washing with water, the regenerated DESs regained a sulfur removal capability of 96.3%, affirming the stability of the DESs.
6 Challenges and Future Perspectives
While DESs exhibit excellent desulfurisation efficiency, larger-scale applications pose challenges in synthesis, viscosity, stability (occasionally), and recovery. DESs precursors, usually cost-effective and easily prepared, raise concerns about temperature and energy usage. In many cases, DESs can be regenerated with water and traditional solvents, maintaining their structure and desulphurisation capabilities. However, after a single extraction stage, DES's effectiveness declines, necessitating recycling, which is impractical on a larger scale. Most literature relies on a basic assumption: model oil (typically dissolving sulphur compounds in n-heptane or n-octane). To validate green solvents' viability, real oil studies are crucial, considering the numerous constituents in actual fuel that may complicate extraction. When scaling from the laboratory to the pilot plant, this aspect is crucial to consider.
DESs for sustainable sulfur removal from fuels are advancing, with research focusing on tailoring compositions to diverse fuel types and sulphur compounds. Integrating catalysts within DES systems enhances desulfurisation kinetics and reduces operating costs. Ensuring economical scalability and efficient DES recovery and recycling methods is imperative for economic viability. As global sulfur emission regulations evolve, future research must ensure DES-based solutions comply. The synergy between chemistry, engineering, and environmental science will drive holistic, eco-friendly solutions for a cleaner and more sustainable energy future.
7 Concluding Remarks
Fuel oil, containing harmful sulfur compounds, poses serious threats to both the environment and human health. Desulfurisation technology plays a crucial role in the oil refining process, aiming to improve fuel quality and protect the environment. The pervasive consequences of sulfur in fuel extend across environmental, health, and economic perspectives. From contributing to particulate matter and acid rain formation to corroding combustion engines, sulfur's detrimental effects are widespread. The toll on human and animal health, coupled with economic burdens tied to environmental degradation, underscores the urgency of adopting measures to mitigate sulfur-related risks. As we move towards sustainable fuel alternatives and advanced technologies, addressing the sulfur challenge becomes pivotal for fostering a healthier, more resilient future.
To maintain sustainability, employing eco-friendly extractants is essential when desulfurising fuel oil. ILs and conventional solvents pose issues, especially regarding toxicity and chemical handling. DESs, with their lower vapour pressure and reduced toxicity, emerge as a safer option. Several Type III DES types, such as glycol-based, sulfolane-based, organic acid-based, and aromatic-based (e.g., toluene and phenol) DESs, show potential as environmentally friendly solvents for efficient sulfur elimination. γ-valerolactone stands out as the best HBA, as DES based on it yields the highest extraction efficiency compared to other salts. Further improvements in desulfurisation performance can be achieved by exploring modifications, such as changing the alkyl chain and experimenting with different anions. Model fuels, composed of aliphatic hydrocarbons and sulfur compounds like Th, DBT, and BT (e.g., n-octane and n-heptane), desulfurise easily. However, the efficiency diminishes when applied to real fuel due to diverse organosulfur compounds. The main interaction mechanisms between sulfur compounds and DES involve π-π interactions and hydrogen bonding. Further investigation into aromatic or aromatic-derived compounds is crucial to enhance the desulfurisation process when designing DESs.
References
IMO (2020) Cutting sulphur oxide emissions. Available: http://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx.
Desai K, Dharaskar S, Khalid M, Gedam V (2022) Effectiveness of ionic liquids in extractive–oxidative desulfurization of liquid fuels: a review. Chem Pap 76:1989–2028. https://doi.org/10.1007/s11696-021-02038-3
Jha D, Maheshwari P, Singh Y et al (2023) A comparative review of extractive desulfurization using designer solvents: Ionic liquids & deep eutectic solvents. J Energy Inst 110:101313. https://doi.org/10.1016/j.joei.2023.101313
Haruna A, Merican Aljunid Merican Z, Gani Musa S, Abubakar S (2022) Sulfur removal technologies from fuel oil for safe and sustainable environment. Fuel 329:125370. https://doi.org/10.1016/j.fuel.2022.125370
Mamuad RY, Choi AES (2023) Biodesulfurization Processes for the Removal of Sulfur from Diesel Oil: A Perspective Report. Energies (Basel) 16:2738. https://doi.org/10.3390/en16062738
Bin LX, Ong W-J (2021) A current overview of the oxidative desulfurization of fuels utilizing heat and solar light: from materials design to catalysis for clean energy. Nanoscale Horiz 6:588–633. https://doi.org/10.1039/D1NH00127B
Malolan R, Gopinath KP, Vo D-VN et al (2021) Green ionic liquids and deep eutectic solvents for desulphurization, denitrification, biomass, biodiesel, bioethanol and hydrogen fuels: a review. Environ Chem Lett 19:1001–1023. https://doi.org/10.1007/s10311-020-01113-7
Sun Y-C, Liu X-N, Wang T-T et al (2019) Green Process for Extraction of Lignin by the Microwave-Assisted Ionic Liquid Approach: Toward Biomass Biorefinery and Lignin Characterization. ACS Sustain Chem Eng 7:13062–13072. https://doi.org/10.1021/acssuschemeng.9b02166
Almashjary KH, Khalid M, Dharaskar S et al (2018) Optimisation of extractive desulfurization using Choline Chloride-based deep eutectic solvents. Fuel 234:1388–1400. https://doi.org/10.1016/j.fuel.2018.08.005
Zhang H, Chen L, Chen Y, Wang Z (2023) Removal of sulfide from fuels by ionic liquids: prospects for the future. Braz J Chem Eng 40:929–963. https://doi.org/10.1007/s43153-023-00304-3
Shan Q, Zhang J, Wang Y, Liu W (2023) Synthesis of Schiff base and its application in adsorption desulphurisation of fuel oil. Int J Environ Anal Chem 103:8428–8440. https://doi.org/10.1080/03067319.2021.1986489
Saeed M, Firdous A, Zaman MS et al (2023) <scp>MOFs</scp> for desulfurization of fuel oil: Recent advances and future insights. J Chin Chem Soc 70:789–824. https://doi.org/10.1002/jccs.202200546
Li A, Song H, Meng H et al (2020) Ultrafast desulfurization of diesel oil with ionic liquid based PMoO catalysts and recyclable NaClO oxidant. Chem Eng J 380:122453. https://doi.org/10.1016/j.cej.2019.122453
Liu W, Jiang W, Zhu W et al (2016) Oxidative desulfurization of fuels promoted by choline chloride-based deep eutectic solvents. J Mol Catal A Chem 424:261–268. https://doi.org/10.1016/j.molcata.2016.08.030
Guan S, Li Z, Xu B et al (2023) Deep eutectic solvents with multiple catalytic sites for highly efficient extractive and oxidative desulfurization. Fuel 333:126329. https://doi.org/10.1016/j.fuel.2022.126329
Jiang Z, Wang X, Deng H et al (2024) Molecular mechanism and experimental study of fuel oil extractive desulfurization technology based on ionic liquid. J Energy Inst 112:101452. https://doi.org/10.1016/j.joei.2023.101452
Li Z, Fu Y, Zhou A, Yang C (2017) Desulfurization of model FCC gasoline by extraction with ionic liquid and conventional extraction solvents. Pet Sci Technol 35:1699–1705. https://doi.org/10.1080/10916466.2017.1358280
Majid MF, Mohd Zaid HF, Kait CF et al (2020) Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J Mol Liq 306:112870. https://doi.org/10.1016/j.molliq.2020.112870
Oke EA, Ijardar SP (2023) Aqueous biphasic systems composed of alcohol-based deep eutectic solvents and inorganic salts: Application in the extraction of dyes with varying hydrophobicity. J Mol Liq 375:121372. https://doi.org/10.1016/j.molliq.2023.121372
Omar KA, Sadeghi R (2023) Database of deep eutectic solvents and their physical properties: A review. J Mol Liq 384:121899. https://doi.org/10.1016/j.molliq.2023.121899
Smith EL, Abbott AP, Ryder KS (2014) Deep Eutectic Solvents (DESs) and Their Applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Oke EA, Ijardar SP (2021) Advances in the application of deep eutectic solvents based aqueous biphasic systems: An up-to-date review. Biochem Eng J 176:108211. https://doi.org/10.1016/j.bej.2021.108211
Saiyasitpanich P, Lu M, Keener TC et al (2005) The Effect of Diesel Fuel Sulfur Content on Particulate Matter Emissions for a Nonroad Diesel Generator. J Air Waste Manage Assoc 55:993–998. https://doi.org/10.1080/10473289.2005.10464685
Luo H, Zhang Q, Niu Y et al (2023) Fine particulate matter and cardiorespiratory health in China: A systematic review and meta-analysis of epidemiological studies. J Environ Sci 123:306–316. https://doi.org/10.1016/j.jes.2022.04.026
Sadare O, Obazu F, Daramola M (2017) Biodesulfurization of Petroleum Distillates—Current Status. Opportunities Future Challenges Environ 4:85. https://doi.org/10.3390/environments4040085
Mohseni E, Hamdi Z, Parvizimehr A, Rahmani A (2023) Adsorptive desulphurisation of benzothiophene and dibenzothiophene from model fuels with modified vermiculite. Int J Environ Anal Chem 103:5691–5705. https://doi.org/10.1080/03067319.2021.1942461
Mahima Kaushik (2018) Science behind smog and its ominous implications. DownToEarth. https://www.downtoearth.org.in/blog/air/science-behind-smog-and-its-ominous-implications-60080
Ashok B, Kumar AN, Jacob A, Vignesh R (2022) Emission formation in IC engines. In: NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines. Elsevier, pp 1–38. https://doi.org/10.1016/B978-0-12-823955-1.00001-2
Gaur N, Sharma S, Yadav N (2024) Environmental pollution. In: Green Chemistry Approaches to Environmental Sustainability. Elsevier, pp 23–41. https://doi.org/10.1016/B978-0-443-18959-3.00010-0
Yin Y, Zhang J, Zhang G (2023) Effect of hygrothermal acid rain environment on the shear bonding performance of CFRP-concrete interface. Constr Build Mater 364:. https://doi.org/10.1016/j.conbuildmat.2022.130002
Abbott AP, Capper G, Davies DL et al (2001) Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem Commun 1:2010–2011. https://doi.org/10.1039/b106357j
Xu P, Zheng G-W, Zong M-H et al (2017) Recent progress on deep eutectic solvents in biocatalysis. Bioresour Bioprocess 4:34. https://doi.org/10.1186/s40643-017-0165-5
Hansen BB, Spittle S, Chen B et al (2021) Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem Rev 121:1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385
Azzouz A, Hayyan M (2023) Potential applications of deep eutectic solvents in nanotechnology: Part II. Chem Eng J 468:143563. https://doi.org/10.1016/j.cej.2023.143563
Zhu A, Bian X, Han W et al (2023) The application of deep eutectic solvents in lithium-ion battery recycling: A comprehensive review. Resour Conserv Recycl 188:106690. https://doi.org/10.1016/j.resconrec.2022.106690
Xiao T, Hou M, Guo X et al (2024) Recent progress in deep eutectic solvent(DES) fractionation of lignocellulosic components : A review. Renew Sustain Energy Rev 192:114243. https://doi.org/10.1016/j.rser.2023.114243
Abbott AP, Barron JC, Ryder KS, Wilson D (2007) Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chem A Europ J 13:6495–6501. https://doi.org/10.1002/chem.200601738
Abranches DO, Coutinho JAP (2023) Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu Rev Chem Biomol Eng 14:141–163. https://doi.org/10.1146/annurev-chembioeng-101121-085323
Oke EA, Oluyinka OA, Afolabi SD et al (2023) Latest insights on technologies for halides and halogenated compounds extraction/abatement from water and wastewater: Challenges and future perspectives. J Water Process Eng 53:103724. https://doi.org/10.1016/j.jwpe.2023.103724
Abbott AP, Capper G, Davies DL et al (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun (Camb) 70–1. https://doi.org/10.1039/b210714g
Prabhune A, Dey R (2023) Green and sustainable solvents of the future: Deep eutectic solvents. J Mol Liq 379:121676. https://doi.org/10.1016/j.molliq.2023.121676
Craveiro R, Aroso I, Flammia V et al (2016) Properties and thermal behavior of natural deep eutectic solvents. J Mol Liq 215:534–540. https://doi.org/10.1016/j.molliq.2016.01.038
Florindo C, Oliveira FS, Rebelo LPN et al (2014) Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain Chem Eng 2:2416–2425. https://doi.org/10.1021/sc500439w
Cui Y, Li C, Yin J et al (2017) Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J Mol Liq 236:338–343. https://doi.org/10.1016/j.molliq.2017.04.052
Song Q-W, He L-N (2019) Atom Economy. Green Chemistry and Chemical Engineering. Springer, New York, New York, NY, pp 3–22
Zhang Q, De Oliveira VK, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108. https://doi.org/10.1039/c2cs35178a
Li C, Zhang J, Li Z et al (2016) Extraction desulfurization of fuels with ‘metal ions’ based deep eutectic solvents (MDESs). Green Chem 18:3789–3795. https://doi.org/10.1039/C6GC00366D
Knyazkov DA, Bolshova TA, Shvartsberg VM et al (2019) Effect of inhibitors on flammability limits of dimethyl ether/air mixtures. Proc Combust Inst 37:4267–4275. https://doi.org/10.1016/j.proci.2018.06.109
Jiang W, Dong L, Liu W et al (2017) Biodegradable choline-like deep eutectic solvents for extractive desulfurization of fuel. Chem Eng Process 115:34–38. https://doi.org/10.1016/j.cep.2017.02.004
Mao C, Zhao R, Li X (2017) Phenylpropanoic acid-based DESs as efficient extractants and catalysts for the removal of sulfur compounds from oil. Fuel 189:400–407. https://doi.org/10.1016/j.fuel.2016.10.113
Shirazinia SR, Semnani A, Nekoeinia M et al (2020) Novel sustainable metal complex based deep eutectic solvents for extractive desulphurisation of fuel. J Mol Liq 301:112364. https://doi.org/10.1016/j.molliq.2019.112364
Hadj-Kali MK, Mulyono S, Hizaddin HF et al (2016) Removal of Thiophene from Mixtures with n-Heptane by Selective Extraction Using Deep Eutectic Solvents. Ind Eng Chem Res 55:. https://doi.org/10.1021/acs.iecr.6b01654
Lima F, Dave M, Silvestre AJD et al (2019) Concurrent Desulfurization and Denitrogenation of Fuels Using Deep Eutectic Solvents. ACS Sustain Chem Eng 7:11341–11349. https://doi.org/10.1021/acssuschemeng.9b00877
Lima F, Gouvenaux J, Branco LC et al (2018) Towards a sulfur clean fuel: Deep extraction of thiophene and dibenzothiophene using polyethylene glycol-based deep eutectic solvents. Fuel 234:414–421. https://doi.org/10.1016/j.fuel.2018.07.043
Shu C, Sun T (2016) Extractive desulfurisation of gasoline with tetrabutyl ammonium chloride-based deep eutectic solvents. Sep Sci Technol 51:1336–1343. https://doi.org/10.1080/01496395.2016.1155602
Chandran D, Khalid M, Walvekar R et al (2019) Deep eutectic solvents for extraction-desulphurization: A review. J Mol Liq 275:312–322. https://doi.org/10.1016/j.molliq.2018.11.051
Fan K, Yang B, Yu S et al (2023) Ternary choline chloride/benzene sulfonic acid/ethylene glycol deep eutectic solvents for oxidative desulfurization at room temperature. RSC Adv 13:25888–25894. https://doi.org/10.1039/D3RA02524A
Xu L, Jia H, Zhu D et al (2022) Hydrogen bonding boosted oxidative desulfurization by ZnCl2/boric acid/polyethylene glycol-based ternary deep eutectic solvents. J Mol Liq 368:120725. https://doi.org/10.1016/j.molliq.2022.120725
Tang X, Zhang Y, Li J et al (2015) Deep Extractive Desulfurization with Arenium Ion Deep Eutectic Solvents. Ind Eng Chem Res 54:4625–4632. https://doi.org/10.1021/acs.iecr.5b00291
Li J, Lei X-J, Tang X-D et al (2019) Acid Dicationic Ionic Liquids as Extractants for Extractive Desulfurization. Energy Fuels 33:4079–4088. https://doi.org/10.1021/acs.energyfuels.9b00307
Zhao X, Zhu G, Jiao L et al (2018) Formation and Extractive Desulfurization Mechanisms of Aromatic Acid Based Deep Eutectic Solvents: An Experimental and Theoretical Study. Chem A Europ J 24:11021–11032. https://doi.org/10.1002/chem.201801631
Makoś P, Boczkaj G (2019) Deep eutectic solvents based highly efficient extractive desulfurization of fuels – Eco-friendly approach. J Mol Liq 296:111916. https://doi.org/10.1016/j.molliq.2019.111916
Guan S, Li Z, Xu B et al (2023) Deep Eutectic Solvents with Excellent Catalytic Ability for Extractive and Oxidative Desulfurization under Room Temperature. ACS Sustain Chem Eng 11:6292–6301. https://doi.org/10.1021/acssuschemeng.2c07647
Sun L, Zhu Z, Su T et al (2019) Novel acidic eutectic mixture as peroxidase mimetics for oxidative desulfurization of model diesel. Appl Catal B 255:. https://doi.org/10.1016/j.apcatb.2019.117747
Yang B, Fan K, Yu S et al (2023) Oxidative desulfurization of thiophene derivatives with L-proline/benzene sulfonic acid deep eutectic solvent and their interaction: An experimental and computational study. J Clean Prod 406:136878. https://doi.org/10.1016/j.jclepro.2023.136878
Jiang W, Li H, Wang C et al (2016) Synthesis of Ionic-Liquid-Based Deep Eutectic Solvents for Extractive Desulfurization of Fuel. Energy Fuels 30:8164–8170. https://doi.org/10.1021/acs.energyfuels.6b01976
Li J, Xiao H, Tang X, Zhou M (2016) Green Carboxylic Acid-Based Deep Eutectic Solvents as Solvents for Extractive Desulfurization. Energy Fuels 30:5411–5418. https://doi.org/10.1021/acs.energyfuels.6b00471
Hao L, Wang M, Shan W et al (2017) L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel. J Hazard Mater 339:216–222. https://doi.org/10.1016/j.jhazmat.2017.06.050
Yang B, Fan K, Yu S et al (2024) Natural deep eutectic solvents of γ-valerolactone and formic acid for oxidative desulfurization with superior efficiency. Fuel 356:129634. https://doi.org/10.1016/j.fuel.2023.129634
Otsuki S, Nonaka T, Takashima N et al (2000) Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 14:1232–1239. https://doi.org/10.1021/ef000096i
Isci A, Kaltschmitt M (2022) Recovery and recycling of deep eutectic solvents in biomass conversions: a review. Biomass Convers Biorefin 12:197–226. https://doi.org/10.1007/s13399-021-01860-9
Thoda O, Svinterikos E, Sakkas KM et al (2023) High-Degree Oxidative Desulfurization of a Commercial Marine Fuel Using Deep Eutectic Solvents and Their Recycling Process. Separations 10:445. https://doi.org/10.3390/separations10080445
Li Z, Liu D, Men Z et al (2018) Insight into effective denitrification and desulfurization of liquid fuel with deep eutectic solvents: an innovative evaluation criterion to filtrate extractants using the compatibility index. Green Chem 20:3112–3120. https://doi.org/10.1039/C8GC00828K
Jiang W, Zhu K, Li H et al (2020) Synergistic effect of dual Brønsted acidic deep eutectic solvents for oxidative desulfurization of diesel fuel. Chem Eng J 394:124831. https://doi.org/10.1016/j.cej.2020.124831
Mao C, Zhao R, Li X, Gao X (2017) Trifluoromethanesulfonic acid-based DESs as extractants and catalysts for removal of DBT from model oil. RSC Adv 7:12805–12811. https://doi.org/10.1039/C6RA28448E
Acknowledgements
The author acknowledges the Research Office of the University of the Witwatersrand for the Centennial Postdoctoral Fellowship granted to him between 2023 and 2025.
Funding
Open access funding provided by University of the Witwatersrand. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The author has no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oke, E.A. Examining the Effectiveness of Deep Eutectic Solvents in Removal of Sulfur from Fuel Oil: A Mini Review. Chemistry Africa (2024). https://doi.org/10.1007/s42250-024-00989-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42250-024-00989-0