Abstract
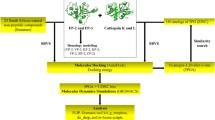
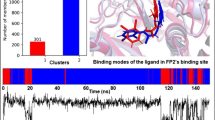
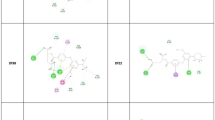
In this work, nine novel thiazole derivatives of substituted benzenesulfonamide carboxylate were designed, synthesized and characterized (1H NMR, 13C NMR and Mass Spectra) for their possible development as antimalarial agents. All synthesized compounds were subjected to molecular docking, drug-likeness, ADMET properties and molecular dynamics studies by in silico methods using Biovia Discovery Studio (DS) 2020 software. The molecular docking study of all the synthesized compounds was carried out against Plasmodium falciparum cysteine protease falcipain 2 (FP-2, 3BPF) and falcipain 3 (FP-3, 3BPM) enzymes using the CDocker program of DS. Further, the best docked compound was studied by molecular dynamics simulation method followed by MM-PBSA calculation. In molecular docking studies, the synthesized thiazolyl benzenesulfonamides exhibited remarkable binding affinity against FP-2 and FP-3 enzymes. Molecular dynamics studies further confirmed the antimalarial potential of the compounds with the formation of well-defined and stable receptor-ligand interactions against both the falcipain enzymes. One derivative, ethyl 4-methyl-2-(4-methyl-2-(4-methylphenylsulfonamido)pentanamido) thiazole-5-carboxylate possesses promising inhibitory potential against both P. falciparum falcipain 2 and falcipain 3 enzymes. Based upon present findings, the thiazolyl benzenesulfonamide-5-carboxylates can be further evaluated for in vitro and in vivo antimalarial effectiveness towards possible development as antimalarial lead molecules and/ or potent antimalarial drug candidates.











Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Stepniewska K, White NJ (2008) Pharmacokinetics determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother 52(5):1589–1596. https://doi.org/10.1128/AAC.00903-07
Nosten F, White NJ (2007) Artemisinin based combination treatment of falciparum malaria. Am J Trop Med Hyg 77(6):181–192
Rudrapal M, Chetia D (2021) Malaria and recent developments in antimalarial drugs. In: Neglected tropical diseases and phytochemicals in drug discovery, pp 499–542. https://doi.org/10.1002/9781119617143.ch21
Eastman RT, Fidock DA (2009) Artemisinin-based combination therapies: a virtual tool in efforts to eliminate malaria. Nat Rev Microbiol 7(12):864–874. https://doi.org/10.1038/nrmicro2239
Shukla M, Rathi K, Hassam M, Yadav DK, Karnatak M, Rawat V, Verma VP (2023) An overview on the antimalarial activity of 1, 2, 4-trioxanes, 1, 2, 4-trioxolanes and 1, 2, 4, 5-tetraoxanes. Med Res Rev. https://doi.org/10.1002/med.21979
Ugwu DI, Okoro UC, Ukoha PO, Gupta A, Okafor SN (2018) Novel anti-inflammatory and analgesic agents: synthesis, molecular docking and in vivo studies. J Enzyme Inhib Med Chem 33(1):405–415. https://doi.org/10.1080/14756366.2018.1426573
Ezugwu JA, Okoro UC, Ezeokonkwo MA, Hariprasad KS, Rudrapal M, Ugwu DI, Gogoi N, Chetia D, Celik I, Ekoh OC (2022) Design, synthesis, molecular docking, molecular dynamics and in vivo antimalarial activity of new dipeptide-sulfonamides. ChemistrySelect 7(5):e202103908. https://doi.org/10.1002/slct.202103908
Sharma I, Sullivan M, McCutchan TF (2015) In vitro antimalarial activity of novel semisynthetic nocathiacin I antibiotics. Antimicrob Agents Chemother 59(6):3174–3179. https://doi.org/10.1128/AAC.04294-14
Sahu S, Ghosh SK, Gahtori P, Pratap Singh U, Bhattacharyya DR, Bhat HR (2019) In silico ADMET study, docking, synthesis and antimalarial evaluation of thiazole-1,3,5-triazine derivatives as Pf-DHFR inhibitor. Pharmacol Rep 71(5):762–767. https://doi.org/10.1016/j.pharep.2019.04.006
Kalita JM, Ghosh SK, Sahu S, Dutta M (2017) Rational design and microwave assisted synthesis of some novel phenyl thiazolyl clubbed s-triazine derivatives as antimalarial antifolate. Futur J Pharm Sci 3(1):11–17. https://doi.org/10.1016/j.fjps.2016.09.004
Ugwuja DI, Okoro UC, Soman SS, Soni R, Okafor SN, Ugwu DI (2019) New peptide derived antimalaria and antimicrobial agents bearing sulphonamide moiety. J Enzyme Inhib Med Chem 34(1):1388–1399. https://doi.org/10.1080/14756366.2019
Ugwu DI, Okoro UC, Ukoha PO, Okafor S, Ibezim A, Kumar NM (2017) Synthesis, characterization, molecular docking and in vitro antimalarial properties of new carboxamides bearing sulphonamide. Eur J Med Chem 135:349–369. https://doi.org/10.1016/j.ejmech.2017.04.029
Ezugwu JA, Okoro UC, Ezeokonkwo MA, Bhimapaka C, Okafor SN, Ugwu DI, Ugwuja DI (2020) Synthesis and biological evaluation of Val-Val dipeptide-sulfonamide conjugates. Arch Pharm 353(7):e2000074. https://doi.org/10.1002/ardp.202000074
Marco M, Coterón JM (2012) Falcipain inhibition as a promising antimalarial target. Curr Top Med Chem 12(5):408–444. https://doi.org/10.2174/156802612799362913
Ettari R, Previti S, Di Chio C, Zappalà M (2021) Falcipain-2 and falcipain-3 inhibitors as promising antimalarial agents. Curr Med Chem 28(15):3010–3031. https://doi.org/10.2174/09298673276662007302153
Rosenthal PJ (2020) Falcipain cysteine proteases of malaria parasites: an update. Biochim Biophys Acta Proteins Proteom 1868(3):140362. https://doi.org/10.1016/j.bbapap.2020
Meng G, Wang M, Zheng A, Dou J, Guo Z (2014) Efficient one-pot synthesis of ethyl 2-substitued-4-methylthiazole-5-carboxylates. Green Chem Lett Rev 7(1):46–49. https://doi.org/10.1080/17518253.2014.895858
Ugwu DI, Ezema BE, Eze FU, Ugwuja DI (2014) Synthesis and structural activity relationship study of antitubercular carboxamides. Int J Med Chem 2014:614808. https://doi.org/10.1155/2014/614808
Ezugwu JA, Okoro UC, Ezeokonkwo MA, Bhimapaka CR, Okafor SN, Ugwu DI, Ekoh OC, Attah SI (2020) Novel Leu-Val based dipeptide as antimicrobial and antimalarial agents: synthesis and molecular docking. Front Chem 8:583926. https://doi.org/10.3389/fchem.2020.583926
Ezugwu JA, Okoro UC, Ezeokonkwo MA, Bhimapaka CR (2020) Synthesis of novel valine-based dipeptide carboxamide bearing benzene sulfonamide moiety as antimalarial agent. Commun Phys Sci. 5(2):176–197
Kalita J, Chetia D, Rudrapal M (2020) Design, synthesis, antimalarial activity and docking study of 7-chloro-4-(2-(substituted benzylidene)hydrazineyl)quinolines. Med Chem 16:928–937. https://doi.org/10.2174/1573406415666190806154722
Ghosh S, Chetia D, Gogoi N, Rudrapal M (2021) Design, molecular docking, drug-likeness, and molecular dynamics studies of 1,2,4-trioxane derivatives as novel Plasmodium falciparum falcipain-2 (FP-2) inhibitors. Biotechnologia 102(3):257–275. https://doi.org/10.5114/bta.2021.108722
Rudrapal M, Banu ZW, Chetia D (2018) Newer series of trioxane derivatives as potent antimalarial agents. Med Chem Res 27(2):653–668. https://doi.org/10.1007/s00044-017-2090-8
Ghorab MM, Soliman AM, Alsaid MS, Askar AA (2020) Synthesis, antimicrobial activity and docking study of some novel 4-(4, 4-dimethyl-2, 6-dioxocyclohexylidene) methylamino derivatives carrying biologically active sulfonamide moiety. Arab J Chem 13(1):545–556. https://doi.org/10.1016/j.arabjc.2017.05.022
Wu G, Robertson DH, Brooks CL, Vieth M (2023) Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J Comput Chem 24(13):1549–1562. https://doi.org/10.1002/jcc.10306
Abdel-Hamid MK, McCluskey A (2014) In silico docking, molecular dynamics and binding energy insights into the bolinaquinone-clathrin terminal domain binding site. Molecules 19(5):6609–6622. https://doi.org/10.3390/molecules19056609
Jain AN (2008) Bias, reporting, and sharing: computational evaluations of docking methods. J Comput Aided Mol Des 22(3–4):201–212. https://doi.org/10.1007/s10822-007-9151-x
Ponnan P, Gupta S, Chopra M, Tandon R, Baghel AS, Gupta G, Prasad AK, Rastogi RC, Bose M, Raj HG (2013) 2D-QSAR, docking studies, and in silico ADMET prediction of polyphenolic acetates as substrates for protein acetyltransferase function of glutamine synthetase of Mycobacterium tuberculosis. ISRN Struct Biol. 2013:1–12. https://doi.org/10.1155/2013/373516
Rudrapal M, Chetia D, Singh V (2017) Novel series of 1,2,4-trioxane derivatives as antimalarial agents. J Enzyme Inhib Med Chem 32(1):1159–1173. https://doi.org/10.1080/14756366.2017
Rudrapal M, Mullapudi S (2019) Design, synthesis, drug-likeness studies and bio-evaluation of some novel chalconeimines. Pharm Chem J 53(9):814–821. https://doi.org/10.1007/s11094-019-02084-y
Kashyap A, Chetia D, Rudrapal M (2016) Synthesis, antimalarial activity evaluation and drug-likeness study of some New Quinoline-Lawsone hybrids. Indian J Pharm Sci 78(6):892–911. https://doi.org/10.4172/pharmaceutical-sciences.1000186
Sharma D, Chetia D, Rudrapal M (2016) Design, synthesis and antimalarial activity of new 2-hydroxy-1,4-naphthoquinone-4-hydroxyanilino hybrid Mannich bases. Asian J Chem 28(4):782–788. https://doi.org/10.14233/ajchem.2016.19478
Lin CH, Chang TT, Sun MF, Chen HY, Tsai FJ, Chang KL, Fisher M, Chen CY (2011) Potent inhibitor design against H1N1 swine influenza: structure-based and molecular dynamics analysis for M2 inhibitors from traditional Chinese medicine database. J Biomol Struct Dyn 28(4):471–482. https://doi.org/10.1080/07391102.2011
Noha SM, Schmidhammer H, Spetea M (2017) Molecular docking, molecular dynamics, and structure-activity relationship explorations of 14-oxygenated N-methylmorphinan-6-ones as potent μ-opioid receptor agonists. ACS Chem Neurosci 8(6):1327–1337. https://doi.org/10.1021/acschemneuro.6b00460
Du QS, Huang RB, Wang SQ, Chou KC (2010) Designing inhibitors of M2 proton channel against H1N1 swine influenza virus. PLoS 5(2):e9388. https://doi.org/10.1371/journal.pone.0009388
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5):449–461. https://doi.org/10.1517/17460441.2015.1032936
Rastelli G, Del Rio A, Degliesposti G, Sgobba M (2010) Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J Comput Chem 31(4):797–810. https://doi.org/10.1002/jcc.21372 (PMID: 19569205)
Acknowledgements
Dr. James Anayochukwu Ezugwu expresses his sincere gratitude to The World Academy of Sciences (TWAS) and the Council for Science and Industrial Research (CSIR), New Delhi for the joint sponsorship of the experimental work at CSIR-India Institute of Chemical Technology, Hyderabad, Telangana, India under the 2017 CSIR-TWAS Postgraduate Fellowship Award (Grant/Award number: 22/FF/CSIR‐TWAS/2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There is no conflict of interest with other people or organizations that could inappropriately influence or bias the content of the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ezugwu, J.A., Okoro, U.C., Ezeokonkwo, M.A. et al. Design, Synthesis, Molecular Docking, Drug-Likeness/ADMET and Molecular Dynamics Studies of Thiazolyl Benzenesulfonamide Carboxylates as Antimalarial Agents. Chemistry Africa (2024). https://doi.org/10.1007/s42250-024-00904-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42250-024-00904-7