Abstract
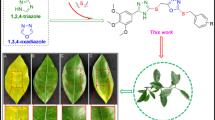
This study focused on efficiently, synthesizing novel derivatives of azo-4-(3H) quinazolinone and azo diamides. Azo diamides derivatives were synthesized by rapidly opening the ring of azo benzoxazine-4-one in the presence of primary aromatic amines, attempts to dehydrate diamides into azo quinazolinones were failed, while azo quinazolinones were synthesized from azo-benzo[d][1,3]oxazin-4-one by using freshly fused sodium acetate in acetic acid The structures of the newly synthesized compounds were characterized using FT-IR, UV–visible, 1H-NMR, and13C-NMR. A solvatochromic UV–Vis absorption study was conducted on the newly synthesized azo quinazolinones using various solvents. The optical band gap of these compounds was subsequently determined. The compounds were then subjected to in vitro anti-microbial screening, measuring their inhibition zones against bacteria (S. aureus, E. coli) and fungus (C. albicans) using well diffusion assays. The newly synthesized azo quinazolinones were also evaluated for their anti-cancer activity against MCF-7 cell lines, revealing moderate to excellent anti-proliferative activity. Additionally, anti-oxidant activity was assessed using the DPPH method and the results revealed that the compounds showed good anti-oxidant properties.















Similar content being viewed by others
Availability of Data and Materials
The data that support this study are available in the article and accompanying online supplementary material.
References
Gupta V, Singh J, Kinger M, Arora AK, Jaswal VS (2015) Synthesis and antiviral activities of some 2, 3-di-substituted quinazoline derivatives. Synthesis 1:2
Mahajan PG, Dige NC, Vanjare BD, Raza H, Hassan M, Seo S-Y, Kim C-H, Lee KH (2019) Facile synthesis of new quinazolinone benzamides as potent tyrosinase inhibitors: Comparative spectroscopic and molecular docking studies. J Mol Struct 1198:126915
Ansari S, Mohammadi-Khanaposhtani M, Asgari MS, Esfahani EN, Biglar M, Larijani B, Rastegar H, Hamedifar H, Mahdavi M, Tas R (2021) Design, synthesis, in vitro and in silico biological assays of new quinazolinone-2-thio-metronidazole derivatives. J Mol Struct 1244:130889
Gatadi S, Pulivendala G, Gour J, Malasala S, Bujji S, Parupalli R, Shaikh M, Godugu C, Nanduri S (2020) Synthesis and evaluation of new 4 (3H)-quinazolinone derivatives as potential anticancer agents. J Mol Struct 1200:127097
Abd El-Dayem NS, Mostafa MA, Hassan SY, Yacout GA, El Sadek MM (2020) Synthesis: antioxidant and antiproliferative activities of novel quinazolinone derivatives. J Appl Chem 13:49–64
Kakoulidou C, Gritzapis PS, Hatzidimitriou AG, Fylaktakidou KC, Psomas G (2020) Zn(II) complexes of (E)-4-(2-(pyridin-2-ylmethylene) hydrazinyl) quinazoline in combination with non-steroidal anti-inflammatory drug sodium diclofenac: structure, DNA binding and photo-cleavage studies, antioxidant activity and interaction with albumin. J Inorg Biochem 211:111194
Xu Z, Zhang Y, Fu H, Zhong H, Hong K, Zhu W (2011) Antifungal quinazolinones from marine-derived Bacillus cereus and their preparation. Bio Med Chem Lett 21(13):4005–4007
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bio Med Chem Lett 26(9):2273–2277
Ran L, Yang H, Luo L, Huang M, Hu D (2020) Discovery of potent and novel quinazolinone sulfide inhibitors with anti-ToCV activity. J Agric Food Chem 68(19):5302–5308
Saul S, Pu S-Y, Zuercher WJ, Einav S, Asquith CR (2020) Potent antiviral activity of novel multi-substituted 4-anilinoquin (az) olines. Bio Med Chem Lett 30(16):127284
Wang M, Zhang G, Wang Y, Wang J, Zhu M, Cen S, Wang Y (2020) Design, synthesis and anti-influenza A virus activity of novel 2, 4-disubstituted quinazoline derivatives. Bio Med Chem Lett 30(11):127143
Zhang G, Wang M, Zhao J, Wang Y, Zhu M, Wang J, Cen S, Wang Y (2020) Design, synthesis and in vitro anti-influenza A virus evaluation of novel quinazoline derivatives containing S-acetamide and NH-acetamide moieties at C-4. Eur J Med Chem 206:112706
Zu G, Gan X, Xie D, Yang H, Zhang A, Li S, Hu D, Song B (2020) Design, synthesis, and anti-ToCV activity of novel 4 (3 H)-quinazolinone derivatives bearing dithioacetal moiety. J Agric Food Chem 68(20):5539–5544
El-Shafey HW, Gomaa RM, El-Messery SM, Goda FE (2020) Quinazoline based HSP90 inhibitors: synthesis, modeling study and ADME calculations towards breast cancer targeting. Bio Med Chem Lett 30(15):127281
Hoan DQ, Hoa LT, Huan TT, Dinh NH (2020) Synthesis and transformation of 4-(1-chloro-1-nitroethyl)-6, 7-dimethoxy-2-methylquinazoline: spectral characterization and anti-cancer properties of some novel quinazoline derivatives. J Het Chem 57(4):1720–1728
Le Y, Gan Y, Fu Y, Liu J, Li W, Zou X, Zhou Z, Wang Z, Ouyang G, Yan L (2020) Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J Enzyme Inhib Med Chem 35(1):555–564
Ramadan SK, Elrazaz EZ, Abouzid KA, El-Naggar AM (2020) Design, synthesis and in silico studies of new quinazolinone derivatives as antitumor PARP-1 inhibitors. RSC Adv 10(49):29475–29492
Yang L, Liu S, Chu J, Miao S, Wang K, Zhang Q, Wang Y, Xiao Y, Wu L, Liu Y (2021) Novel anilino quinazoline-based EGFR tyrosine kinase inhibitors for treatment of non-small cell lung cancer. Biomater Sci 9(2):443–455
Noser AA, El-Naggar M, Donia T, Abdelmonsef AH (2020) Synthesis, in silico and in vitro assessment of new quinazolinones as anticancer agents via potential AKT inhibition. Mol 25(20):4780
Du H, Ding M, Luo N, Shi J, Huang J, Bao X (2021) Design, synthesis, crystal structure and in vitro antimicrobial activity of novel 1, 2, 4-triazolo [1, 5-a] pyrimidine-containing quinazolinone derivatives. Mol Div 25:711–722
Norouzbahari M, Salarinejad S, Güran M, Şanlıtürk G, Emamgholipour Z, Bijanzadeh HR, Toolabi M, Foroumadi A (2020) Design, synthesis, molecular docking study, and antibacterial evaluation of some new fluoroquinolone analogues bearing a quinazolinone moiety. DARU J Pharm Sci 28:661–672
Xu Z (2020) 1,2,3-triazole-containing hybrids with potential antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur J Med Chem 206:112686
Le-Nhat-Thuy G, Thi NN, Pham-The H, Thi TAD, Thi HN, Thi THN, Hoang SN, Van Nguyen T (2020) Synthesis and biological evaluation of novel quinazoline-triazole hybrid compounds with potential use in Alzheimer’s disease. Bio Med Chem Lett 30(18):127404
Prinsloo IF, Zuma NH, Aucamp J, N’Da DD (2021) Synthesis and in vitro antileishmanial efficacy of novel quinazolinone derivatives. Chem Biol Drug Des 97(2):383–398
Sharma M, Chauhan K, Shivahare R, Vishwakarma P, Suthar MK, Sharma A, Gupta S, Saxena JK, Lal J, Chandra P (2013) Discovery of a new class of natural product-inspired quinazolinone hybrid as potent antileishmanial agents. J Med Chem 56(11):4374–4392
Eskikanbur S, Sayin K, Kose M, Zengin H, McKee V, Kurtoglu M (2015) Synthesis of two new azo-azomethines; spectral characterization, crystal structures, computational and fluorescence studies. J Mol Struct 1094:183–194
Herbst W, Hunger K (2006) Industrial organic pigments: production, properties, applications. Wiley, New York
Kirk RE, Othmer DF, Grayson M, Eckroth D (1985) Kirk-Othmer concise encyclopedia of chemical technology. (No Title)
Lazar T (2005) Industrial dyes—chemistry, properties, applications. Wiley Online Library, New York
Zollinger H (2003) Color chemistry: syntheses, properties, and applications of organic dyes and pigments. Wiley, New York
Benkhaya S, M'rabet S, El Harfi A (2020) Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6(1)
Favre-Besse F-C, Poirel O, Bersot T, Kim-Grellier E, Daumas S, El Mestikawy S, Acher FC, Pietrancosta N (2014) Design, synthesis and biological evaluation of small-azo-dyes as potent vesicular glutamate transporters inhibitors. Eur J Med Chem 78:236–247
Mi Y, Zhang J, Han X, Tan W, Miao Q, Cui J, Li Q, Guo Z (2021) Modification of carboxymethyl inulin with heterocyclic compounds: synthesis, characterization, antioxidant and antifungal activities. Int J Biol Macromol 181:572–581
Singh K, Pal R, Khan SA, Kumar B, Akhtar MJ (2021) Insights into the structure activity relationship of nitrogen-containing heterocyclics for the development of antidepressant compounds: an updated review. J Mol Struct 1237:130369
Patel DR, Patel KC (2010) Synthesis, characterization and application of quinazolinone based reactive dyes for various fibers. Fiband Pol 11:537–544
Levy PR, Stephen H (1956) Syntheses in the quinazolone series. Part I. Synthesis of 2: 3-diaryl-4-quinazolones. J Chem Soc 205:985–988
Patel DR, Patel KC (2011) Synthesis and characterization of reactive dyes based on 2-phenyl-3-[4′-(4″-aminophenylsulphonamido)] phenyl-4 (3H)-quinazolinone-6-sulphonic acid. Arab J Chem 4(3):279–285
Patel M, Patel V (1987) Azo dyes with 4-quinazolinone ring for dyeing nylon, wool, cotton and viscose rayon fibres. Ind J Tex Res 12:3–16
Fried B, Sherma J (1982) Thin-layer chromatography: techniques and applications
Kareem Samad M (2017) Synthesis, characterization and dying performance studies of some azo dyes derived from m-phenylenediamine. Zanco J Pure Appl Sci 28(6):148–157
Ayoob MM, Hawaiz FE (2022) Design, synthesis, molecular docking ADMET and anti-bacterial activities of some new benzamides and their corresponding quinazolinone derivatives. Egypt J Chem 65(132):1517–1530
El-Sawy AA, Mohamed SK, Eissa AE-MM, Tantawy AH, Issac YA (2012) Synthesis, reactions and biological evaluation of pentadecanyl benzoxazinone and pentadecanyl quinazolinone derivatives. J Chem Pharm Res 4(5):2755–2762
Ayoob M, Hawaiz F, Dege N, Kansız S (2023) Structural investigation and hirshfeld surface analysis of N-(3-chloro-4-methylphenyl)-2-(3-nitrobenzamido) benzamide. J Str Chem 64(6):1049–1058
Al-Majidi SM, Al-tamimy HM (2017) Synthesis, identification and surface active properties of some nonionic surfactants containing quinazolinone ring. J Appl Chem 10(5):37–46
Banerjee M, Behera CC, Pradhan GC, Azam MA, Sahu SK (2009) Synthesis and biological evaluation of some anthranilic acid and 2-phenylquinazoline-4 (3H)-one analogues. S Af J Chem 62:134–142
Madkour H (2004) Reactivity of 4H-3, 1-benzoxazin-4-ones towards nitrogen and carbon nucleophilic reagents: applications to the synthesis of new heterocycles. Ark 1:36–54
Mohammed SJ, Salih AK, Rashid MAM, Omer KM, Abdalkarim KA (2020) Synthesis, spectroscopic studies and keto-enol tautomerism of novel 1,3,4-thiadiazole derivative containing 3-mercaptobutan-2-one and quinazolin-4-one moieties. Mol 25(22):5441
Sumathy A, Palanisamy S (2017) Synthesis of some novel quinazoline derivatives having anti-cancer activity. Int J Pharm Pharm Sci 3(2):48–52
Rajput CS, Singhal S (2013) Synthesis, characterization, and anti inflamatory activity of newer quinazolinone analogs. J Pharm 2013:907525
Ayoob MM, Hussein AJ, Samad MK, Dege N, Hawaiz FE, Mohamed SK, Hussain FH (2021) Synthesis, anti-bacterial and anti-oxidant activity of azo-oxazolone and their ring opening azo-benzamide derivatives. Curr Org Synth 18(5):493–505
Ayoob MM, Hawaiz FE, Hussein A, Samad MK, Hussain F, Mohamed SK (2020) Synthesis, spectroscopic investigation, anti-bacterial and antioxidant activites of some new azo-benzofuran derivatives. Egypt J Chem 63(7):2617–2629
Jiménez-Estrada M, Velázquez-Contreras C, Garibay-Escobar A, Sierras-Canchola D, Lapizco-Vázquez R, Ortiz-Sandoval C, Burgos-Hernández A, Robles-Zepeda RE (2013) In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complement Altern Med 13:1–8
López-Romero JC, Torres-Moreno H, Rodríguez-Martínez KL, Ramírez-Audelo V, Vidal-Gutiérrez M, Hernández J, Robles-Zepeda RE, Ayala-Zavala JF, González-Ríos H, Valenzuela-Melendres M (2022) Fouquieria splendens: a source of phenolic compounds with antioxidant and antiproliferative potential. Eur J Integr Med 49:102084
Charitos G, Trafalis DT, Dalezis P, Potamitis C, Sarli V, Zoumpoulakis P, Camoutsis C (2019) Synthesis and anticancer activity of novel 3,6-disubstituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives. Arab J Chem 12(8):4784–4794
El-Hashash MAE-AM, Salem MS, Al-Mabrook SAM (2018) Synthesis and anticancer activity of novel quinazolinone and benzamide derivatives. Res Chem Intermed 44:2545–2559
Desai N, Dodiya AM (2014) Synthesis, characterization and antimicrobial screening of quinoline based quinazolinone-4-thiazolidinone heterocycles. Arab J Chem 7(6):906–913
Rakesh K, Kumara H, Ullas B, Shivakumara J, Gowda DC (2019) Amino acids conjugated quinazolinone-Schiff’s bases as potential antimicrobial agents: synthesis, SAR and molecular docking studies. Bio Chem 90:103093
Ramilo-Gomes F, Addis Y, Tekamo I, Cavaco I, Campos DL, Pavan FR, Gomes CS, Brito V, Santos AO, Domingues F (2021) Antimicrobial and antitumor activity of S-methyl dithiocarbazate Schiff base zinc(II) complexes. J Inorg Biochem 216:111331
Al-Obaid A, Abdel-Hamide S, El-Kashef H, Abdel-Aziz A, El-Azab A, Al-Khamees H, El-Subbagh H (2009) Synthesis, in vitro antitumor activity and molecular modelling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur J Med Chem 44:2379–2391
Al-Omary FA, Abou-Zeid LA, Nagi MN, Habib E-SE, Alaa A-M, El-Azab AS, Abdel-Hamide SG, Al-Omar MA, Al-Obaid AM, El-Subbagh HI (2010) Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg Med Chem 18(8):2849–2863
Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Youssef KM, Al-Obaid AM, El-Subbagh HI (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4 (3H)-quinazolinone analogs. Bioorg Med Chem 14(24):8608–8621
Airinei A, Homocianu M, Dorohoi DO (2010) Changes induced by solvent polarity in electronic absorption spectra of some azo disperse dyes. J Mol Liq 157(1):13–17
Zakerhamidi M, Ahmadi-Kandjani S, Moghadam M, Ortyl E, Kucharski S (2011) Substituent and solvent effects on the dipole moments and photophysical properties of two azo sulfonamide dyes. J Mol Struct 996(1–3):95–100
Kakanejadifard A, Azarbani F, Zabardasti A, Kakanejadifard S, Ghasemian M, Esna-ashari F, Omidi S, Shirali S, Rafieefar M (2013) The synthesis, structural characterization and antibacterial properties of some 2-((4-amino-1, 2, 5-oxadiazol-3-ylimino) methyl)-4-(phenyldiazenyl) phenol. Dyes Pigm 97(1):215–221
Ghasemian M, Kakanejadifard A, Azarbani F, Zabardasti A, Kakanejadifard S (2014) Spectroscopy and solvatochromism studies along with antioxidant and antibacterial activities investigation of azo–azomethine compounds 2-(2-hydroxyphenylimino) methyl)-4-phenyldiazenyl) phenol. Spectrochim Acta A Mol Biomol 124:153–158
Kakanejadifard A, Azarbani F, Zabardasti A, Rezayat A, Ghasemian M, Kakanejadifard S (2013) Spectroscopic and solvatochromism studies along with antioxidant and antibacterial activities investigation of 2-((2-mercaptophenylimino) methyl)-4-(phenyldiazenyl) phenol. Spectrochim Acta A Mol Biomol 114:404–409
Khanmohammadi H, Abdollahi A (2012) New diaminomaleonitrile-based azo-azomethine dyes; synthesis, characterization and spectral properties. Dyes Pigm 94(1):163–168
Kumar N, Mahadevan K, Nagaraju G (2020) Development and detection of level II and III features of latent fingerprints using highly sensitive AIE based coumarin fluorescent derivative. J Sci Adv Mater Devi 5(4):520–526
Manjunatha B, Bodke YD (2021) Novel isoxazolone based azo dyes: synthesis, characterization, computational, solvatochromic UV–Vis absorption and biological studies. J Mol Struct 1244:130933
Nagaraju G, Shubha J, Manjunath K, Dupont J (2018) Ionothermal synthesis of TiO2 nanoparticles for enhanced photocatalytic H2 generation. Int J Hydrog 43(8):4028–4035
Samad MK, Hawaiz FE (2019) Synthesis, characterization, antioxidant power and acute toxicity of some new azo-benzamide and azo-imidazolone derivatives with in vivo and in vitro antimicrobial evaluation. Bio Chem 85:431–444
Ahmed D, Khan MM, Saeed R (2015) Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antiox 4(2):394–409
Idhayadhulla A, Xia L, Lee YR, Kim SH, Wee Y-J, Lee C-S (2014) Synthesis of novel and diverse mollugin analogues and their antibacterial and antioxidant activities. Bio Chem 52:77–82
Allam HA, Aly EE, Farouk AK, El Kerdawy AM, Rashwan E, Abbass SE (2020) Design and synthesis of some new 2,4,6-trisubstituted quinazoline EGFR inhibitors as targeted anticancer agents. Bio Chem 98:103726
Haredi Abdelmonsef A, Eldeeb Mohamed M, El-Naggar M, Temairk H, Mohamed Mosallam A (2020) Novel quinazolin-2,4-dione hybrid molecules as possible inhibitors against malaria: synthesis and in silico molecular docking studies. Fron Mol Biosci 7:105
Metwally NH, Abdelrazek FM, Eldaly SM (2018) Synthesis, molecular docking, and biological evaluation of some novel bis-heterocyclic compounds based N,N′-([1,1′-biphenyl]-4,4′-diyl)bis(2-cyanoacetamide) as potential anticancer agents. J Heterocycl Chem 55(12):2668–2682
Plaeyao K, Kampangta R, Korkokklang Y, Talodthaisong C, Saenchoopa A, Thammawithan S, Latpala K, Patramanon R, Kayunkid N, Kulchat S (2023) Gingerol extract-stabilized silver nanoparticles and their applications: colorimetric and machine learning-based sensing of Hg(II) and antibacterial properties. RSC Adv 13(29):19789–19802
Hemalatha K, Madhumitha G, Ravi L, Khanna VG, Al-Dhabi NA, Arasu MV (2016) Binding mode of dihydroquinazolinones with lysozyme and its antifungal activity against Aspergillus species. J Photochem Photobiol B Biol 161:71–79
Acknowledgements
This work was supported by Salahaddin University-Erbil, Erbil, Kurdistan-Iraq as the PhD program (No. 3/1/5/1520 at 6/10/2020). The authors would like to thank Dr. Mohammed K. Samad, Dr. Aveen F. Jalal and Dr. Rebwar Muhammad Hamasalih for the excellent technical assistance performed in vitro biochemical assays.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayoob, M.M., Hawaiz, F.E. Novel 4(3H)Quinazolinones Based Azo Dyes: Synthesis, Investigations, Solvatochromic UV–Vis Absorption, Antioxidant and Biological Assessments. Chemistry Africa 7, 623–642 (2024). https://doi.org/10.1007/s42250-023-00803-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00803-3