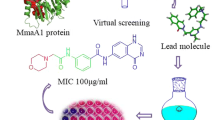
Abstract
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) remains a major global health threat. The treatment of TB is hampered by the emergence of multidrug resistance, so there is an urgent need to discover new anti-tubercular agents. Multi-target anti-tubercular agents targeting key proteins involved in mycolic acid biosynthesis represent an effective approach to combat TB. This study used a multi-target computational approach to probe the inhibitory potential of 18 cytosporone E analogues against vital proteins involved in Mtb mycolic acid synthesis (InhA, KasA, and MmpL3) utilizing the Schrodinger suite. Among these, 17 cytosporone E derivatives displayed docking scores ranging from − 8.677 to − 4.617 kcal/mol, which were better than the reference TLM6 (− 3.477 kcal/mol) in KasA. While 7 compounds (1–7) showed higher binding affinity (− 12.418 to − 10.103 kcal/mol) than the InhA co-crystallized ligand AP-124 (− 9.866 kcal/mol) and significant binding (− 9.647 to − 7.279 kcal/mol) against MmpL3. The reference ligand SQ109 showed the highest docking score (− 12.786 kcal/mol) in MmpL3. The seven shortlisted compounds showed acceptable MM-GBSA free binding energy against the three proteins. Further, compounds 1–4 were studied by molecular dynamics (MD) simulations for 100 n and density functional theory (DFT) calculations. Compounds 1–4 and protein showed an average RMSD below 3 Å, reflecting the stability of the compounds with InhA protein. The compounds’ order of increased reactivity and photo-stability according to the DFT data are as follows 1 > 3 > 2 > 4. Also, compounds 1–4 showed favorable ADMET properties (absorption, distribution, metabolism, excretion, and toxicity). Thus, these compounds may be considered for further experimental testing to confirm their potential anti-tubercular activity.









Similar content being viewed by others
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, and excretion
- CADD:
-
Compute aided drug design
- EMA:
-
European Medical Agency
- FDA:
-
Food and Drug Administration
- InhA:
-
Enoyl-acyl protein reductase
- KasA:
-
Beta-ketoacyl-ACP synthase A
- MD:
-
Molecular dynamics
- MDR-TB:
-
Multidrug resistance tuberculosis
- MM-GBSA:
-
Molecular Mechanics Generalized Born and surface area
- MMpL:
-
Mycobacterial Member Protein Large
- Mtb :
-
Mycobacterium tuberculosis
- PPW:
-
Protein preparation wizard
- RMSD:
-
Root mean square deviation
- RMSF:
-
Root mean square fluctuation
- TB:
-
Tuberculosis
- TDM:
-
Trehalose dimycolate
- TMM:
-
Trehalose monomycolate
- XDR-TB:
-
Extensively drug resistance tuberculosis
- XP:
-
Extra precision
References
Perveen S, Sharma R (2022) Screening approaches and therapeutic targets: the two driving wheels of tuberculosis drug discovery. Biochem Pharmacol 197:114906. https://doi.org/10.1016/j.bcp.2021.114906
Kingdon ADH, Alderwick LJ (2021) Structure-based in silico approaches for drug discovery against Mycobacterium tuberculosis. Comput Struct Biotechnol J 19:3708–3719. https://doi.org/10.1016/j.csbj.2021.06.034
Aragaw WW, Lee BM, Yang X, Zimmerman MD, Gengenbacher M, Dartois V, Chui WK, Jackson CJ, Dick T (2021) Potency boost of a Mycobacterium tuberculosis dihydrofolate reductase inhibitor by multienzyme F420H2-dependent reduction. Proc Natl Acad Sci USA 118:1–10. https://doi.org/10.1073/pnas.2025172118
Mendes V, Green SR, Evans JC, Hess J, Blaszczyk M, Spry C, Bryant O, Cory-Wright J, Chan DSH, Torres PHM, Wang Z, Nahiyaan N, O’Neill S, Damerow S, Post J, Bayliss T, Lynch SL, Coyne AG, Ray PC, Abell C, Rhee KY, Boshoff HIM, Barry CE, Mizrahi V, Wyatt PG, Blundell TL (2021) Inhibiting Mycobacterium tuberculosis CoaBC by targeting an allosteric site. Nat Commun 12:1–12. https://doi.org/10.1038/s41467-020-20224-x
Lee YV, Choi SB, Wahab HA, Lim TS, Choong YS (2019) Applications of ensemble docking in potential inhibitor screening for Mycobacterium tuberculosis isocitrate lyase using a local plant database. J Chem Inf Model 59:2487–2495. https://doi.org/10.1021/acs.jcim.8b00963
Ntie-Kang F, Kannan S, Wichapong K, Owono Owono LC, Sippl W, Megnassan E (2014) Binding of pyrazole-based inhibitors to Mycobacterium tuberculosis pantothenate synthetase: docking and MM-GB(PB)SA analysis. Mol Biosyst 10:223–239. https://doi.org/10.1039/c3mb70449a
Song L, Merceron R, Gracia B, Quintana AL, Risseeuw MDP, Hulpia F, Cos P, Aínsa JA, Munier-Lehmann H, Savvides SN, Van Calenbergh S (2018) Structure guided lead generation toward nonchiral M. tuberculosis thymidylate kinase inhibitors. J Med Chem 61:2753–2775. https://doi.org/10.1021/acs.jmedchem.7b01570
Njogu PM, Guantai EM, Pavadai E, Chibale K (2016) Computer-aided drug discovery approaches against the tropical infectious diseases malaria, tuberculosis, trypanosomiasis, and leishmaniasis. ACS Infect Dis 2:8–31. https://doi.org/10.1021/acsinfecdis.5b00093
Deb PK, Al-Shar’i NA, Venugopala KN, Pillay M, Borah P (2021) In vitro anti-TB properties, in silico target validation, molecular docking and dynamics studies of substituted 1,2,4-oxadiazole analogues against Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 36:869–884. https://doi.org/10.1080/14756366.2021.1900162
Koch O, Jäger T, Heller K, Khandavalli PC, Pretzel J, Becker K, Flohé L, Selzer PM (2013) Identification of M. tuberculosis thioredoxin reductase inhibitors based on high-throughput docking using constraints. J Med Chem 56:4849–4859. https://doi.org/10.1021/jm3015734
Sharma A, De Rosa M, Singla N, Singh G, Barnwal RP, Pandey A (2021) Tuberculosis: an overview of the immunogenic response, disease progression, and medicinal chemistry efforts in the last decade toward the development of potential drugs for extensively drug-resistant tuberculosis strains. J Med Chem 64:4359–4395. https://doi.org/10.1021/acs.jmedchem.0c01833
Ruddraraju KV, Aggarwal D, Niu C, Baker EA, Zhang RY, Wu L, Zhang ZY (2020) Highly POTENT AND SELECTIve N-Aryl oxamic acid-based inhibitors for Mycobacterium tuberculosis protein tyrosine phosphatase B. J Med Chem 63:9212–9227. https://doi.org/10.1021/acs.jmedchem.0c00302
Bhakhar KA, Gajjar ND, Bodiwala KB, Sureja DK, Dhameliya TM (2021) Identification of anti-mycobacterial agents against mmpL3: virtual screening, ADMET analysis and MD simulations. J Mol Struct 1244:130941. https://doi.org/10.1016/j.molstruc.2021.130941
Kamsri P, Hanwarinroj C, Phusi N, Pornprom T, Chayajarus K, Punkvang A, Suttipanta N, Srimanote P, Suttisintong K, Songsiriritthigul C, Saparpakorn P, Hannongbua S, Rattanabunyong S, Seetaha S, Choowongkomon K, Sureram S, Kittakoop P, Hongmanee P, Santanirand P, Chen Z, Zhu W, Blood RA, Takebayashi Y, Hinchliffe P, Mulholland AJ, Spencer J, Pungpo P (2020) Discovery of new and potent InhA inhibitors as antituberculosis agents: structure-based virtual screening validated by biological assays and X-ray crystallography. J Chem Inf Model 60:226–234. https://doi.org/10.1021/acs.jcim.9b00918
Cellitti SE, Shaffer J, Jones DH, Mukherjee T, Gurumurthy M, Bursulaya B, Boshoff HI, Choi I, Nayyar A, Lee YS, Cherian J, Niyomrattanakit P, Dick T, Manjunatha UH, Barry CE, Spraggon G, Geierstanger BH (2012) Structure of Ddn, the deazaflavin-dependent nitroreductase from Mycobacterium tuberculosis involved in bioreductive activation of PA-824. Structure 20:101–112. https://doi.org/10.1016/j.str.2011.11.001
Lee BM, Harold LK, Almeida DV, Afriat-Jurnou L, Aung HL, Forde BM, Hards K, Pidot SJ, Ahmed FH, Mohamed AE, Taylor MC, West NP, Stinear TP, Greening C, Beatson SA, Nuermberger EL, Cook GM, Jackson CJ (2020) Predicting nitroimidazole antibiotic resistance mutations in Mycobacterium tuberculosis with protein engineering. PLoS Pathog 16:1–27. https://doi.org/10.1371/journal.ppat.1008287
Esposito S, Bianchini S, Blasi F (2015) Bedaquiline and delamanid in tuberculosis. Expert Opin Pharmacother 16:2319–2330. https://doi.org/10.1517/14656566.2015.1080240
Muhammad Dayyab F, Iliyasu G, Garba Ahmad B, Aliyu Umar I, Musa Shuaib N, Bajehson M, Muhammad Daiyab I, Akpala O, Remilekun O, Garba Habib A (2022) Emerging threat of drug-resistant tuberculosis and trends in the era of COVID-19: a descriptive study from northwestern Nigeria. J Clin Tuberc Other Mycobact Dis 28:100319. https://doi.org/10.1016/j.jctube.2022.100319
Souza LLL, dos Santos FL, de Crispim JA, Fiorati RC, Dias S, Bruce ATI, Alves YM, Ramos ACV, Berra TZ, da Costa FBP, Alves LS, Monroe AA, Fronteira I, Arcêncio RA (2021) Causes of multidrug-resistant tuberculosis from the perspectives of health providers: challenges and strategies for adherence to treatment during the COVID-19 pandemic in Brazil. BMC Health Serv Res 21:1–10. https://doi.org/10.1186/s12913-021-07057-0
Suppli CH, Norman A, Folkvardsen DB, Gissel TN, Weinreich UM, Koch A, Wejse C, Lillebaek T (2022) First outbreak of multidrug-resistant tuberculosis (MDR-TB) in Denmark involving six Danish-born cases. Int J Infect Dis 117:258–263. https://doi.org/10.1016/j.ijid.2022.02.017
Priyadarshini N, Veeramani A, Chinnathambi P, Palanichamy A, Al-Dosary MA, Ali MA, Lee J, Paulraj B (2022) Antimycobacterial effect of plant derived phthalate against Mycobacterium tuberculosis H37Ra. Physiol Mol Plant Pathol 117:101761. https://doi.org/10.1016/j.pmpp.2021.101761
Baptista R, Bhowmick S, Shen J, Mur LAJ (2021) Molecular docking suggests the targets of anti-mycobacterial natural products. Molecules 26:1–14. https://doi.org/10.3390/molecules26020475
Ferlazzo G, Mohr E, Laxmeshwar C, Hewison C, Hughes J, Jonckheere S, Khachatryan N, De Avezedo V, Egazaryan L, Shroufi A, Kalon S, Cox H, Furin J, Isaakidis P (2018) Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis 18:536–544. https://doi.org/10.1016/S1473-3099(18)30100-2
Kim CT, Kim TO, Shin HJ, Ko YC, Choe YH, Kim HR, Kwon YS (2018) Bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis: a multicentre cohort study in Korea. Eur Respir J 51:1–10. https://doi.org/10.1183/13993003.02467-2017
Padmapriyadarsini C, Vohra V, Bhatnagar A, Solanki R, Sridhar R, Anande L, Muthuvijaylakshmi M (2022) Bedaquiline, delamanid, linezolid, and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin Infect Dis. https://doi.org/10.1093/cid/ciac528
Bolla JR (2020) Targeting MmpL3 for anti-tuberculosis drug development. Biochem Soc Trans 48:1463–1472. https://doi.org/10.1042/BST20190950
Su CC, Klenotic PA, Cui M, Lyu M, Morgan CE, Yu EW (2021) Structures of the mycobacterial membrane protein MmpL3 reveal its mechanism of lipid transport. PLoS Biol 19:1–23. https://doi.org/10.1371/journal.pbio.3001370
Durairaj DR, Shanmughavel P (2019) In silico drug design of thiolactomycin derivatives against Mtb-KasA enzyme to inhibit multidrug resistance of Mycobacterium tuberculosis. Interdiscip Sci Comput Life Sci 11:215–225. https://doi.org/10.1007/s12539-017-0257-0
Dupont C, Chen Y, Xu Z, Roquet-Banères F, Blaise M, Witt AK, Dubar F, Biot C, Guérardel Y, Maurer FP, Chng SS, Kremer L (2019) A piperidinol-containing molecule is active against Mycobacterium tuberculosis by inhibiting the mycolic acid flippase activity of MmpL3. J Biol Chem 294:17512–17523. https://doi.org/10.1074/jbc.RA119.010135
Sylvia CK, Luckner R, Machutta CA, Tonge PJ (2009) Crystal structures of Mycobacterium tuberculosis KasA show mode of action within cell wall biosynthesis and its inhibition by thiolactomycin. Structure 17:1–20. https://doi.org/10.1038/nature08365.Reconstructing
Marrakchi H, Lanéelle MA, Daffé M (2014) Mycolic acids: structures, biosynthesis, and beyond. Chem Biol 21:67–85. https://doi.org/10.1016/j.chembiol.2013.11.011
Schiebel J, Kapilashrami K, Fekete A, Bommineni GR, Schaefer CM, Mueller MJ, Tonge PJ, Kisker C (2013) Structural basis for the recognition of mycolic acid precursors by KasA, a condensing enzyme and drug target from Mycobacterium tuberculosis. J Biol Chem 288:34190–34204. https://doi.org/10.1074/jbc.M113.511436
Shilpi JA, Ali MT, Saha S, Hasan S, Gray AI, Seidel V (2015) Molecular docking studies on InhA, MabA and PanK enzymes from Mycobacterium tuberculosis of ellagic acid derivatives from Ludwigia adscendens and Trewia nudiflora. Silico Pharmacol 3:1–7. https://doi.org/10.1186/s40203-015-0014-1
Grover S, Engelhart CA, Pérez-Herrán E, Li W, Abrahams KA, Papavinasasundaram K, Bean JM, Sassetti CM, Mendoza-Losana A, Besra GS, Jackson M, Schnappinger D (2021) Two-way regulation of MmpL3 expression identifies and validates inhibitors of MmpL3 function in Mycobacterium tuberculosis. ACS Infect Dis 7:141–152. https://doi.org/10.1021/acsinfecdis.0c00675
Sabbah M, Mendes V, Vistal RG, Dias DMG, Záhorszká M, Mikušová K, Korduláková J, Coyne AG, Blundell TL, Abell C (2020) Fragment-based design of mycobacterium tuberculosis inha inhibitors. J Med Chem 63:4749–4761. https://doi.org/10.1021/acs.jmedchem.0c00007
Vilchèze C, Wang F, Arai M, Hazbón MH, Colangeli R, Kremer L, Weisbrod TR, Alland D, Sacchettini JC, Jacobs WR (2006) Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat Med 12:1027–1029. https://doi.org/10.1038/nm1466
Shaw DJ, Robb K, Vetter BV, Tong M, Molle V, Hunt NT, Hoskisson PA (2017) Disruption of key NADH-binding pocket residues of the Mycobacterium tuberculosis InhA affects DD-CoA binding ability. Sci Rep 7:1–7. https://doi.org/10.1038/s41598-017-05042-4
Slayden RA, Barry CE (2002) The role of KasA and KasB in the biosynthesis of meromycolic acids and isoniazid resistance in Mycobacterium tuberculosis. Tuberculosis 82:149–160. https://doi.org/10.1054/tube.2002.0333
Li W, Obregón-Henao A, Wallach JB, North EJ, Lee RE, Gonzalez-Juarrero M, Schnappinger D, Jackson M (2016) Therapeutic potential of the Mycobacterium tuberculosis mycolic acid transporter, MmpL3. Antimicrob Agents Chemother 60:5198–5207. https://doi.org/10.1128/AAC.00826-16
McNeil MB, O’Malley T, Dennison D, Shelton CD, Sunde B, Parish T (2020) Multiple mutations in Mycobacterium tuberculosis MmpL3 increase resistance to MmpL3 inhibitors. MSphere 5:1–7. https://doi.org/10.1128/msphere.00985-20
Degiacomi G, Benjak A, Madacki J, Boldrin F, Provvedi R, Palù G, Kordulakova J, Cole ST, Manganelli R (2017) Essentiality of mmpL3 and impact of its silencing on Mycobacterium tuberculosis gene expression. Sci Rep 7:1–8. https://doi.org/10.1038/srep43495
Li W, Upadhyay A, Fontes FL, North EJ, Wang Y, Crans DC, Grzegorzewicz AE, Jones V, Franzblau SG, Lee RE, Crick DC, Jackson M (2014) Novel insights into the mechanism of inhibition of MmpL3, a target of multiple pharmacophores in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:6413–6423. https://doi.org/10.1128/AAC.03229-14
Su CC, Klenotic PA, Bolla JR, Purdy GE, Robinson CV, Yu EW (2019) MmpL3 is a lipid transporter that binds trehalose monomycolate and phosphatidylethanolamine. Proc Natl Acad Sci USA 166:11241–11246. https://doi.org/10.1073/pnas.1901346116
Adams O, Deme JC, Parker JL, Fowler PW, Lea SM, Newstead S (2021) Cryo-EM structure and resistance landscape of M. tuberculosis MmpL3: an emergent therapeutic target. Structure 29:1182-1191.e4. https://doi.org/10.1016/j.str.2021.06.013
Almeida C, Kehraus S, Prudêncio M, König GM (2011) Marilones A-C, phthalides from the sponge-derived fungus Stachylidium sp.,. Beilstein J Org Chem 7:1636–1642. https://doi.org/10.3762/bjoc.7.192
Kan WLT, Cho CH, Rudd JA, Lin G (2008) Study of the anti-proliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J Ethnopharmacol 120:36–43. https://doi.org/10.1016/j.jep.2008.07.027
Ibraheem W, Chaar C, Camiade E, Hervé V, Fouquenet D, Roux A-E, Si-Tahar M, Ahmed E, Thibonnet J, Thiery E, Petrignet J (2022) Synthesis, antibacterial and cytotoxic evaluation of cytosporone E and analogs. J Mol Struct 1252:132135. https://doi.org/10.1016/j.molstruc.2021.132135
Beau J, Mahid N, Burda WN, Harrington L, Shaw LN, Mutka T, Kyle DE, Barisic B, Van Olphen A, Baker BJ (2012) Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar Drugs 10:762–774. https://doi.org/10.3390/md10040762
Ramakrishnan SP, Suresh AJ (2020) Insights into comparative molecular docking study of selected novel thiophene derivative vs standard anti-tubercular drugs against Mycobacterium tuberculosis target enzymes. AIP Conf Proc 2277:120005–120007. https://doi.org/10.1063/5.0027187
Vickers CF, Silva APG, Chakraborty A, Fernandez P, Kurepina N, Saville C, Naranjo Y, Pons M, Schnettger LS, Gutierrez MG, Park S, Kreiswith BN, Perlin DS, Thomas EJ, Cavet JS, Tabernero L (2018) Structure-based design of MptpB inhibitors that reduce multidrug-resistant Mycobacterium tuberculosis survival and infection burden in vivo. J Med Chem 61:8337–8352. https://doi.org/10.1021/acs.jmedchem.8b00832
Ejalonibu MA, Ogundare SA, Elrashedy AA, Ejalonibu MA, Lawal MM, Mhlongo NN, Kumalo HM (2021) Drug discovery for mycobacterium tuberculosis using structure-based computer-aided drug design approach. Int J Mol Sci 22:1–39. https://doi.org/10.3390/ijms222413259
Tian S, Wang J, Li Y, Li D, Xu L, Hou T (2015) The application of in silico drug-likeness predictions in pharmaceutical research. Adv Drug Deliv Rev 86:2–10. https://doi.org/10.1016/j.addr.2015.01.009
Aleksandrov A, Myllykallio H (2019) Advances and challenges in drug design against tuberculosis: application of in silico approaches. Expert Opin Drug Discov 14:35–46. https://doi.org/10.1080/17460441.2019.1550482
Sundar S, Thangamani L, Manivel G, Kumar P, Piramanayagam S (2019) Molecular docking, molecular dynamics and MM/PBSA studies of FDA approved drugs for protein kinase a of Mycobacterium tuberculosis; application insights of drug repurposing. Informat Med Unlock 16:100210. https://doi.org/10.1016/j.imu.2019.100210
Koseki Y, Aoki S (2014) Computational medicinal chemistry for rational drug design: identification of novel chemical structures with potential anti-tuberculosis activity. Curr Top Med Chem 14:176–188. https://doi.org/10.2174/1568026613666131113155042
Jin Y, Fan S, Lv G, Meng H, Sun Z, Jiang W, Steven G, Musterman M, Placeholder P, Musterman M (2017) Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking. Open Chem 15:299–307. https://doi.org/10.1515/chem-2017-0039
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3:935–949. https://doi.org/10.1038/nrd1549
De Vivo M, Masetti M, Bottegoni G, Cavalli A (2016) Role of molecular dynamics and related methods in drug discovery. J Med Chem 59:4035–4061. https://doi.org/10.1021/acs.jmedchem.5b01684
Mohammed AO, Abo-Idrees MI, Makki AA, Ibraheem W, Alzain AA (2022) Drug repurposing against main protease and RNA-dependent RNA polymerase of SARS-CoV-2 using molecular docking, MM-GBSA calculations and molecular dynamics. Struct Chem. https://doi.org/10.1007/s11224-022-01999-9
Alzain AA, Elbadwi FA (2021) Identification of novel TMPRSS2 inhibitors for COVID-19 using e-pharmacophore modelling, molecular docking, molecular dynamics and quantum mechanics studies. Inform Med Unlock 26:100758. https://doi.org/10.1016/j.imu.2021.100758
Omer SE, Ibrahim TM, Krar OA, Ali AM, Makki AA, Ibraheem W, Alzain AA (2022) Drug repurposing for SARS-CoV-2 main protease: Molecular docking and molecular dynamics investigations. Biochem Biophys Rep 29:101225. https://doi.org/10.1016/j.bbrep.2022.101225
Sánchez-Sixto C, Prazeres VFV, Castedo L, Lamb H, Hawkins AR, González-Bello C (2005) Structure-based design, synthesis, and biological evaluation of inhibitors of Mycobacterium tuberculosis type II dehydroquinase. J Med Chem 48:4871–4881. https://doi.org/10.1021/jm0501836
Rohilla A, Khare G, Tyagi AK (2018) A combination of docking and cheminformatics approaches for the identification of inhibitors against 4′ phosphopantetheinyl transferase of: Mycobacterium tuberculosis. RSC Adv 8:328–341. https://doi.org/10.1039/c7ra11198c
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759. https://doi.org/10.1021/jm030644s
Alzain AA, Ismail A, Fadlelmola M, Mohamed MA, Mahjoub M, Makki AA, Elsaman T (2022) De novo design of novel spike glycoprotein inhibitors using e-pharmacophore modeling, molecular hybridization, ADMET, quantum mechanics and molecular dynamics studies for COVID-19. Pak J Pharm Sci 35:313–321. https://doi.org/10.3672/PJPS.2022.35.1.SUP.313-321.1
Halder SK, Mim M, Alif H (2022) Oxa-376 and Oxa-530 variants of β-lactamase: computational study uncovers potential therapeutic targets of Acinetobacter baumannii. RSC Adv 12:24319–24338. https://doi.org/10.1039/d2ra02939a
Alzain AA, Elbadwi FA, Alsamani FO (2022) Discovery of novel TMPRSS2 inhibitors for COVID-19 using in silico fragment-based drug design, molecular docking, molecular dynamics, and quantum mechanics studies. Inform Med Unlock 29:100870. https://doi.org/10.1016/J.IMU.2022.100870
Osman EA, Abdalla MA, Abdelraheem MO, Ali MF, Osman SA, Tanir YM, Abdelrahman M, Ibraheem W, Alzain AA (2021) Design of novel coumarins as potent Mcl-1 inhibitors for cancer treatment guided by 3D-QSAR, molecular docking and molecular dynamics. Inform Med Unlock 26:100765. https://doi.org/10.1016/j.imu.2021.100765
Umare MD, Khedekar PB, Chikhale RV (2021) Mycobacterial membrane protein large 3 (MmpL3) inhibitors: a promising approach to combat tuberculosis. ChemMedChem 16:3136–3148. https://doi.org/10.1002/cmdc.202100359
Yuanita E, Sudirman NKT, Dharmayani M, Ulfa JS (2020) Quantitative structure–activity relationship (QSAR) and molecular docking of xanthone derivatives as anti-tuberculosis agents. J Clin Tuberc Other Mycobact Dis 21:100203. https://doi.org/10.1016/j.jctube.2020.100203
Raghu MS, Pradeep Kumar CB, Yogesh Kumar K, Prashanth MK, Alshahrani MY, Ahmad I, Jain R (2022) Design, synthesis and molecular docking studies of imidazole and benzimidazole linked ethionamide derivatives as inhibitors of InhA and antituberculosis agents. Bioorgan Med Chem Lett 60:128604. https://doi.org/10.1016/j.bmcl.2022.128604
Venugopala KN, Chandrashekharappa S, Deb PK, Tratrat C, Pillay M, Chopra D, Al-Shar’i NA, Hourani W, Dahabiyeh LA, Borah P, Nagdeve RD, Nayak SK, Padmashali B, Morsy MA, Aldhubiab BE, Attimarad M, Nair AB, Sreeharsha N, Haroun M, Shashikanth S, Mohanlall V, Mailavaram R (2021) Anti-tubercular activity and molecular docking studies of indolizine derivatives targeting mycobacterial InhA enzyme. J Enzyme Inhib Med Chem 36:1472–1487. https://doi.org/10.1080/14756366.2021.1919889
Verma R, Boshoff HIM, Arora K, Bairy I, Tiwari M, Varadaraj BG, Shenoy GG (2020) Synthesis, evaluation, molecular docking, and molecular dynamics studies of novel N-(4-[pyridin-2-yloxy]benzyl)arylamine derivatives as potential antitubercular agents. Drug Dev Res 81:315–328. https://doi.org/10.1002/ddr.21623
Bhoi MN, Borad MA, Jethava DJ, Acharya PT, Pithawala EA, Patel CN, Pandya HA, Patel HD (2019) Synthesis, biological evaluation and computational study of novel isoniazid containing 4H-Pyrimido[2,1-b]benzothiazoles derivatives. Eur J Med Chem 177:12–31. https://doi.org/10.1016/j.ejmech.2019.05.028
Trawally M, Demir-Yazıcı K, İpek Dingiş-Birgül S, Kaya K, Akdemir A, Güzel-Akdemir Ö (2022) Mandelic acid-based spirothiazolidinones targeting M. tuberculosis: synthesis, in vitro and in silico investigations. Bioorg Chem 121:105688. https://doi.org/10.1016/j.bioorg.2022.105688
Kuo MR, Morbidoni HR, Alland D, Sneddon SF, Gourlie BB, Staveski MM, Leonard M, Gregory JS, Janjigian AD, Yee C, Musser JM, Kreiswirth B, Iwamoto H, Perozzo R, Jacobs WR, Sacchettini JC, Fidock DA (2003) Targeting tuberculosis and malaria through inhibition of enoyl reductase. Compound activity and structural data. J Biol Chem 278:20851–20859. https://doi.org/10.1074/jbc.M211968200
Xia Y, Zhou Y, Carter DS, McNeil MB, Choi W, Halladay J, Berry PW, Mao W, Hernandez V, O’Malley T, Korkegian A, Sunde B, Flint L, Woolhiser LK, Scherman MS, Gruppo V, Hastings C, Robertson GT, Ioerger TR, Sacchettini J, Tonge PJ, Lenaerts AJ, Parish T, Alley MRK (2018) Discovery of a cofactor-independent inhibitor of Mycobacterium tuberculosis InhA. Life Sci Alliance 1:1–12. https://doi.org/10.2650/lsa.201800025
Shaikh MS, Kanhed AM, Chandrasekaran B, Palkar MB, Agrawal N, Lherbet C, Hampannavar GA, Karpoormath R (2019) Discovery of novel N-methyl carbazole tethered rhodanine derivatives as direct inhibitors of Mycobacterium tuberculosis InhA. Bioorgan Med Chem Lett 29:2338–2344. https://doi.org/10.1016/j.bmcl.2019.06.015
Mahfuz AMUB, Stambuk Opazo F, Aguilar LF, Iqbal MN (2022) Carfilzomib as a potential inhibitor of NADH-dependent enoyl-acyl carrier protein reductases of Klebsiella pneumoniae and Mycobacterium tuberculosis as a drug target enzyme: insights from molecular docking and molecular dynamics. J Biomol Struct Dyn 40:4021–4037. https://doi.org/10.1080/07391102.2020.1852966
Zhang B, Li J, Yang X, Wu L, Zhang J, Yang Y, Zhao Y, Zhang L, Yang X, Yang X, Cheng X, Liu Z, Jiang B, Jiang H, Guddat LW, Yang H, Rao Z (2019) Crystal structures of membrane transporter MmpL3, an anti-TB drug target. Cell 176:636-648.e13. https://doi.org/10.1016/j.cell.2019.01.003
Kb S, Kumari A, Shetty D, Fernandes E, Dv C, Jays J, Murahari M (2020) Journal of Molecular Graphics and Modelling Structure based pharmacophore modelling approach for the design of azaindole derivatives as DprE1 inhibitors for tuberculosis. J Mol Graph Model 101:107718. https://doi.org/10.1016/j.jmgm.2020.107718
Honório KM, Moda TL, Andricopulo AD (2013) Pharmacokinetic properties and in silico ADME modeling in drug discovery pharmacokinetic properties and in silico ADME modeling in drug discovery. Med Chem (Los Angeles) 9:163–176. https://doi.org/10.2174/1573406411309020002
Li AP (2001) Screening for human ADME/Tox drug properties in drug discovery. Drug Discov Today 6:357–366. https://doi.org/10.1016/S1359-6446(01)01712-3
Priest BT, Bell IM, Garcia ML (2008) Role of hERG potassium channel assays in drug development. Channels 2:87–93. https://doi.org/10.4161/chan.2.2.6004
Volynets GP, Starosyla SA, Rybak MY, Bdzhola VG, Kovalenko OP, Vdovin VS, Yarmoluk SM, Tukalo MA (2019) Dual-targeted hit identification using pharmacophore screening. J Comput Aid Mol Des 33:955–964. https://doi.org/10.1007/s10822-019-00245-5
Kumari M, Singh R, Subbarao N (2021) Exploring the interaction mechanism between potential inhibitor and multi-target Mur enzymes of mycobacterium tuberculosis using molecular docking, molecular dynamics simulation, principal component analysis, free energy landscape, dynamic cross-correlation matrices, vector movements, and binding free energy calculation. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1989040
Kumari M, Subbarao N (2022) Identification of novel multitarget antitubercular inhibitors against mycobacterial peptidoglycan biosynthetic Mur enzymes by structure-based virtual screening. J Biomol Struct Dyn 40:8185–8196. https://doi.org/10.1080/07391102.2021.1908913
Kovalenko OP, Volynets GP, Rybak MY, Starosyla SA, Gudzera OI, Lukashov SS, Bdzhola VG, Yarmoluk SM, Boshoff HI, Tukalo MA (2019) Dual-target inhibitors of mycobacterial aminoacyl-tRNA synthetases among N-benzylidene- N ′-thiazol-2-yl-hydrazines. Medchemcomm 10:2161–2169. https://doi.org/10.1039/c9md00347a
Acknowledgements
We acknowledge Mme Katia Dekimeche from Schrodinger for the technical support and help.
Funding
No funding has been acquired for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alzain, A.A., Makki, A.A. & Ibraheem, W. Insights into the Inhibition of Mycolic Acid Synthesis by Cytosporone E Derivatives for Tuberculosis Treatment Via an In Silico Multi-target Approach. Chemistry Africa 6, 1811–1831 (2023). https://doi.org/10.1007/s42250-023-00605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00605-7