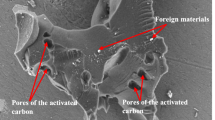
Abstract
Recently, increasing the discharge of untreated dyes containing wastewater is causing a serious environmental problem in water systems. Textile industries generate a huge volume of wastewater that contains many pollutants such as suspended solids, acidity, alkalinity, dissolved solids, sulfate, chromium, COD, and different types of dyes including Congo red (CR). Normally, CR is mainly associated with many industries like paint production, paper manufacturing, and garment printing. Therefore, this study aimed to investigate the removal of CR from aqueous and textile wastewater using activated carbon developed from corn cobs under four adsorption factors named pH, adsorbent dose, contact time, and initial dye concentration. This activated carbon was developed from corn cobs using pyrolysis and the chemical activating agent. Carbonization was done using an electrical fume furnace at 500 °C and then treated with phosphoric acid at a ratio of 1:1 (w/v). The textile wastewater properties were described by the color of 1200 (pt-co), pH 9, BOD5 200 mg/L, COD 370 mg/L, and TDS 4970 mg/L. The corn cob activated carbon (CCAC) was characterized by ash 2.4%, moisture 4.9%, volatile 40.9%, fixed carbon 51.8%, carbon yield 59.3%, bulk density 290 mg/m3, and surface area 650 m2/g. FTIR results showed the presence of multi-functional groups such as hydroxyl, alcohol, ketone, pyrone, aliphatic, ether, and aromatic whereas the X-ray analysis indicated the amorphous nature of carbonaceous matter. The maximum CR removal was obtained at the experimental condition of pH 6.2, adsorbent dose 1.6, contact time 84 min, and initial dye concentration of 90 mg/L in conventional experiments studies whereas the highest dye removals were found to be 96% from the aqueous solution and 88% from real textile wastewater under the factorial approach. Langmuir isothermal and pseudo-second-order at R2 0.997 are in good agreement with experimental adsorption data. Finally, CCAC is a good adsorbent for the discoloration of dyes from textile wastewater with the potential to be scaled up.











Similar content being viewed by others
Data Availability
All data are fully available without restriction.
References
Afolalu SA, Ikumapayi OM, Ogedengbe TS, Kazeem RA, Ogundipe AT (2022) Waste pollution, wastewater and effluent treatment methods – An overview. Mater Today Proc. https://doi.org/10.1016/j.matpr.2022.04.231
Mukherjee A, Goswami N, Dhak D (2022) Photocatalytic remediation of industrial dye waste streams using biochar and metal-biochar hybrids: a critical review. Chem Afr. https://doi.org/10.1007/s42250-022-00467-5
Lin L, Yang H, Xu X (2022) Effects of water pollution on human health and disease heterogeneity: a review. Front Environ Sci. https://doi.org/10.3389/fenvs.2022.880246
Lebron YAR, Moreira VR, Maia A, Couto CF, Moravia WG, Amaral MCS (2021) Integrated photo-fenton and membrane-based techniques for textile effluent reclamation. Sep Purif Technol 272:118932
Ravikumar M, Bedewibilal (2018) Removal of congo red from dye wastewater using adsorption. Int J Eng Tech 4(1):18–27
Jamee R, Siddique R (2019) Biodegradation of synthetic dyes of textile effluent by microorganisms: an environmentally and economically sustainable approach. Eur J Microbiol Immunol 9(4):114–118. doi: https://doi.org/10.1556/1886.2019.00018
Panda B, Mondal D, Mandal S, Khatun J, Mukherjee A, Dhak D (2022) One-pot solution combustion synthesis of porous spherical-shaped magnesium zinc binary oxide for efficient fluoride removal and photocatalytic degradation of methylene blue and Congo red dye. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-22551-6
Khurana K (2018) An overview of textile and apparel business advances in Ethiopia. Res J Text Appar 22(3):212–223. doi: https://doi.org/10.1108/RJTA-01-2018-0003
Ismail M et al (2019) Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr Pharm Des 25(34):3645–3663. https://doi.org/10.2174/1381612825666191021142026
Abdul-Husain SA, Alramahi S (2021) Removal of pathogenic pollutants using electrocoagulation using aluminium electrodes. IOP Conf Ser Mater Sci Eng 1184(1):012011. doi: https://doi.org/10.1088/1757-899x/1184/1/012011
Chandran D (2015) A review of the textile industries waste water treatment methodologies. Int J Sci Eng Res 7(1):392–403
Liu Q (2020) Pollution and treatment of dye waste-water. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/514/5/052001
Mat Yashim M, Sheikh Abdullah SR, Razali N, Nizam Salleh MS, Wan Osman WH (2016) Removal of reactive dye from artificial textile effluent by corncob: an agro solid waste. Appl Mech Mater 699:141–145. https://doi.org/10.4028/www.scientific.net/amm.699.141
Adane T, Adugna AT, Alemayehu E (2021) Textile industry effluent treatment techniques. J Chem. https://doi.org/10.1155/2021/5314404
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17(1):145–155. doi: https://doi.org/10.1007/s10311-018-0785-9
Achparaki M et al (2012) “We are IntechOpen, the world ’ s leading publisher of Open Access books Built by scientists, for scientists TOP 1%.” Intech 13
Alam MS (2015) Removal of congo red dye from industrial wastewater by untreated sawdust. Am J Environ Prot 4(5):207. https://doi.org/10.11648/j.ajep.20150405.12
Sirajudheen P, Nikitha MR, Karthikeyan P, Meenakshi S (2020) Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene oxide–carboxymethyl cellulose recyclable composite: Adsorption investigation of parametric studies and their mechanisms. Surf Interfaces 21:100648. doi: https://doi.org/10.1016/j.surfin.2020.100648
Neolaka YAB et al (2020) The adsorption of cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): study of kinetics, isotherms and thermodynamics. J Mater Res Technol 9(3):6544–6556. doi: https://doi.org/10.1016/j.jmrt.2020.04.040
Lee JE, Park YK (2020) Applications of modified biochar-based materials for the removal of environment pollutants: a mini review. Sustain. https://doi.org/10.3390/su12156112
Fito J, Abrham S, Angassa K (2020) Adsorption of methylene blue from textile industrial wastewater onto activated carbon of parthenium hysterophorus. Int J Environ Res 14(5):501–511. https://doi.org/10.1007/s41742-020-00273-2
Xiao Y, Lyu H, Yang C, Zhao B, Wang L, Tang J (2021) Graphitic carbon nitride/biochar composite synthesized by a facile ball-milling method for the adsorption and photocatalytic degradation of enrofloxacin. J Environ Sci 103:93–107. https://doi.org/10.1016/j.jes.2020.10.006
Tebeje A, Worku Z, Nkambule TTI, Fito J (2021) Adsorption of chemical oxygen demand from textile industrial wastewater through locally prepared bentonite adsorbent. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03230-4
Fito J, Kefeni KK, Nkambule TTI (2022) The potential of biochar-photocatalytic nanocomposites for removal of organic micropollutants from wastewater. Sci Total Environ 829:154648. https://doi.org/10.1016/j.scitotenv.2022.154648
Vikrant K et al (2018) Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.10.193
El-Baz A, Hendy I, Dohdoh A, Srour M (2020) Adsorption technique for pollutants removal; current new trends and future challenges – A review. Egypt J Eng Sci Technol 32(1):1–24. https://doi.org/10.21608/eijest.2020.45536.1015
Abu-Nada A, Abdala A, McKay G (2021) Removal of phenols and dyes from aqueous solutions using graphene and graphene composite adsorption: a review. J Environ Chem Eng 9(5):105858. https://doi.org/10.1016/j.jece.2021.105858
Kassahun E, Tibebu S, Tadesse Y, Awish N (2022) “Synthesis optimization of activated carbon driven from scrap tire for adsorbent yield and methylene blue removal under response surface methodology.” Adv Mater Sci Eng 2022
Ho S (2020) Removal of dyes from wastewater by adsorption onto activated carbon: mini review. J Geosci Environ Prot 08:120–131. https://doi.org/10.4236/gep.2020.85008
Bhadauria N, Suresh A (2020) “Adsorptive Removal of congo red dye from wastewater using fenugreek powder. https://doi.org/10.20944/PREPRINTS202004.0173.V1
Wong S et al (2020) Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci Rep 10(1):1–13. doi: https://doi.org/10.1038/s41598-020-60021-6
Jabar JM, Odusote YA, Alabi KA, Ahmed IB (2020) Kinetics and mechanisms of congo-red dye removal from aqueous solution using activated Moringa oleifera seed coat as adsorbent. Appl Water Sci 10(6):1–11. doi: https://doi.org/10.1007/s13201-020-01221-3
Baker MM, Nuno DB (2021) Socio-Economic determinants of maize production of smallholder farmers in Eastern Oromia, Ethiopia. Grassroots J Nat Resour 4(1):29–39. https://doi.org/10.33002/nr2581.6853.040103
Wondim D, Tefera T, Tesfaye Y (2020) Determinants of Maize market supply, production and marketing constraints: the case of Dembecha District, West Gojjam Zone, Ethiopia. Int J Econ Energy Environ 5(5):83. https://doi.org/10.11648/j.ijeee.20200505.13
van Dijk M, Morley T, van Loon M, Reidsma P, Tesfaye K, van Ittersum MK (2020) Reducing the maize yield gap in Ethiopia: decomposition and policy simulation. Agric Syst 183:102828. https://doi.org/10.1016/j.agsy.2020.102828
Buah W (2016) Conversion of corn cobs waste into activated carbons for adsorption of heavy metals from minerals processing wastewater. Int J Environ Prot Policy 4(4):98. https://doi.org/10.11648/j.ijepp.20160404.11
Thongkrua S, Suriya P (2022) Removal of colour and COD in biologically pre-treated leachate using activated carbon from corn cobs. Pollution 8(2):657–670. https://doi.org/10.22059/POLL.2022.328345.1153
Mopoung S, Moonsri P, Palas W, Khumpai S (2015) Characterization and Properties of activated carbon prepared from tamarind seeds by KOH activation for Fe(III) adsorption from aqueous solution. Sci World J. https://doi.org/10.1155/2015/415961
Wirnkor VA (2019) Removal of biochemical and chemical oxygen demands in Vegetable oil industry effluents using adsorbents from fluted pumpkin mesocarp. Int J Adv Res Chem Sci 6(11):1–11. https://doi.org/10.20431/2349-0403.0611001
Ojedokun AT, Bello OS (2017) Liquid phase adsorption of Congo red dye on functionalized corn cobs. J Dispers Sci Technol 38(9):1285–1294. https://doi.org/10.1080/01932691.2016.1234384
Tian C, Feng C, Wei M, Wu Y (2018) Enhanced adsorption of anionic toxic contaminant Congo Red by activated carbon with electropositive amine modification. Chemosphere 208:476–483. https://doi.org/10.1016/j.chemosphere.2018.06.005
Nethaji S, Sivasamy A, Mandal AB (2013) Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of cr(VI). Bioresour Technol 134:94–100. https://doi.org/10.1016/j.biortech.2013.02.012
Amran F, Zaini MAA (2021) Sodium hydroxide-activated Casuarina empty fruit: Isotherm, kinetics and thermodynamics of methylene blue and congo red adsorption. Environ Technol Innov 23:101727. https://doi.org/10.1016/j.eti.2021.101727
Lafi R, Montasser I, Hafiane A (2019) Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt Sci Technol 37:1–2. https://doi.org/10.1177/0263617418819227
Das A, Mondal S, Hansda KM, Adak MK, Dhak D (2022) A critical review on the role of carbon supports of metal catalysts for selective catalytic hydrogenation of chloronitrobenzenes. Appl Catal A Gen. https://doi.org/10.1016/j.apcata.2022.118955
Togibasa O, Mumfaijah M, Allo YK, Dahlan K, Ansanay YO (2021) The effect of chemical activating agent on the properties of activated carbon from sago waste. Appl Sci. https://doi.org/10.3390/app112411640
Yang Z, Gleisner R, Mann DH, Xu J, Jiang J, Zhu JY (2020) Lignin based activated carbon using h3po4 activation. Polym (Basel) 12(12):1–16. https://doi.org/10.3390/polym12122829
Waktole Y, Seid B, Mereta T, Fufa F (2019) Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory. Appl Water Sci 9(2):1–10. https://doi.org/10.1007/s13201-019-0906-z
Yehuala G, Worku Z, Angassa K, Nkambule TTI, Fito J (2021) Electrochemical degradation of chemical oxygen demand in the textile industrial wastewater through the modified electrodes. Arab J Sci Eng. https://doi.org/10.1007/s13369-021-05776-4
Bedada D, Angassa K, Tiruneh A, Kloos H, Fito J (2020) Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol Environ 5(3):184–195. https://doi.org/10.1007/s40974-020-00160-8
Moges A, Nkambule TTI, Fito J (2022) The application of GO-Fe 3 O 4 nanocomposite for chromium adsorption from tannery industry wastewater. J Environ Manage 305:114369. https://doi.org/10.1016/j.jenvman.2021.114369
Baird RB, Eaton AD, Rice EW (2017) Standard Methods for the examination of water and wastewater
Ayoola W, Oyetunji A, Baba YD (2019) Proximate, ultimate analysis and industrial applications of some Nigerian coals. ABUAD J Eng Res Dev 2(1):20–25
Zięzio M, Charmas B, Jedynak K, Hawryluk M, Kucio K (2020) Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid (V). Appl Nanosci 10(12):4703–4716
Shah K, Parmar A (2018) “Characterization and properties of activated carbon prepared from tamarind seeds,” 03: 1–4
Mukherjee A, Dhak P, Dhak D (2022) The solvothermal synthesis of a 3D rod-like Fe–Al bimetallic metal–organic-framework for efficient fluoride adsorption and photodegradation of water-soluble carcinogenic dyes. Environ Sci Adv 1(2):121–137. doi: https://doi.org/10.1039/d1va00019e
Dungani R, Sofyan S, Tati M, Jamaludin K (2022) “Study of Characterization of activated carbon from coconut shells on various particle scales as filler agent in composite materials,” 50(4): 256–271
Mukherjee A et al (2019) Efficient fluoride removal and dye degradation of Contaminated water using Fe/Al/Ti Oxide nanocomposite. ACS Omega 4:9686–9696. https://doi.org/10.1021/acsomega.9b00252
Alam MZ, Bari MN, Kawsari S (2022) Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environ Sustain Indic. https://doi.org/10.1016/j.indic.2022.100176
Ghosh RK, Ray DP, Debnath S, Tewari A, Das I (2019) Optimization of process parameters for methylene blue removal by jute stick using response surface methodology. Environ Prog Sustain Energy 38(5):620–634. https://doi.org/10.1002/ep.13146
Chen X, Tian Z, Cheng H, Xu G, Zhou H (2021) Adsorption process and mechanism of heavy metal ions by different components of cells, using yeast (Pichia pastoris) and Cu2 + as biosorption models. RSC Adv 11(28):17080–17091. https://doi.org/10.1039/d0ra09744f
Gour DK (2021) “Physicochemical studies, characterization & adsorption capacity of activated carbon derived from dathura stronsium fruit shell. Indian J Environ Eng 1(1):1–4. https://doi.org/10.54105/ijee.a1801.051121
Bayisa YM, Bullo TA, Akuma DA (2021) Chromium removal from tannery effluents by adsorption process via activated carbon chat stems (Catha edulis) using response surface methodology. BMC Res Notes 14(1):1–6. https://doi.org/10.1186/s13104-021-05855-7
Hamza NAE (2013) Adsorption of Metals (fe(II), cr(III) and Co(II)) from aqueous solution by using activated carbon prepared from Mesquite Tree. Sci J Anal Chem. https://doi.org/10.11648/j.sjac.20130102.12
Adhikari B, Pokharel, Gurung V (2019) Preparation and characterization of activated carbon from walnut (Jaglansregia) shells by chemical activation with zinc chloride (ZnCl2). J Appl Sci 7(11):1124–1129
Eke-emezie N, Etuk BR, Akpan OP, Chinweoke OC (2022) Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl Water Sci 12(7):1–12. https://doi.org/10.1007/s13201-022-01679-3
Raupp ÍN, Filho AV, Arim AL, Muniz ARC, da Rosa GS (2021) Development and characterization of activated carbon from olive pomace: experimental design, kinetic and equilibrium studies in nimesulide adsorption. Mater (Basel). https://doi.org/10.3390/ma14226820
Njewa JB, Vunain E, Biswick T (2022) Synthesis and Characterization of Activated Carbons Prepared from Agro-Wastes by Chemical Activation. J Chem. https://doi.org/10.1155/2022/9975444
Gebrezgiher M, Kiflie Z (2020) Utilization of cactus peel as biosorbent for the removal of reactive dyes from textile dye effluents. J Environ Public Health. https://doi.org/10.1155/2020/5383842
Altalhi T et al (2021) Integrated approach in treatment of solid olive residue and olive wastewater. Mater Res Express. https://doi.org/10.1088/2053-1591/ac34b9
Rind IK, Memon N, Khuhawar MY, Soomro WA, Lanjwani MF (2022) Modeling of cadmium(II) removal in a fixed bed column utilizing hydrochar-derived activated carbon obtained from discarded mango peels. Sci Rep 12(1):1–15. https://doi.org/10.1038/s41598-022-11574-1
Sheikhi M, Rezaei H (2021) Adsorption of hexavalent chromium ions from aqueous solutions using nano-chitin: kinetic, isotherms and thermodynamic studies. Water Pract Technol 16(2):436–451. https://doi.org/10.2166/wpt.2021.007
Lingamdinne LP et al (2022) Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 286:131776. https://doi.org/10.1016/j.chemosphere.2021.131776
Xiao W et al (2021) Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.124773
Yakout SM, Hassan MR, El-Zaidy ME, Shair OH, Salih AM (2019) Kinetic study of methyl orange adsorption on activated carbon derived from pine (Pinus strobus) sawdust. BioResources. https://doi.org/10.15376/biores.14.2.4560-4574
Hu JP, Liu Y, Bi HM, You FY, Xie PT (2016) Adsorption of methylene blue from aqueous solution by AB-8 macroreticular resin. MATEC Web Conf 60(12):230–232. https://doi.org/10.1051/matecconf/20166002002
Meko RL (2021) Adsorption of reactive dyes from textile wastewater using corn stalk activated carbon. Res Artic 11:2021
Aminu I, Gumel SM, Ahmad WA, Idris AA (2020) Adsorption isotherms and kinetic studies of congo-red removal from waste water using activated carbon prepared from jujube seed. Am J Anal Chem 11(01):47–59. https://doi.org/10.4236/ajac.2020.111004
Njewa JB, Biswick TT, Vunain E, Lagat CS, Lugasi SO (2022) Synthesis and characterization of activated carbon from agrowastes for the removal of acetic acid from an aqueous solution. Adsorpt Sci Technol. https://doi.org/10.1155/2022/7701128
Zhang L et al (2018) Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv 8(74):42280–42291. https://doi.org/10.1039/c8ra08990f
Khelifi O, Affoune AM, Nacef M, Chelaghmia ML, Laksaci H (2022) Response surface modeling and optimization of Ni(II) and Cu(II) Ions competitive adsorption capacity by sewage sludge activated carbon. Arab J Sci Eng 47(5):5797–5809. https://doi.org/10.1007/s13369-021-05534-6
Acknowledgements
We would like to acknowledge Addis Ababa Science and Technology University for its laboratory facilities.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, YT, JF, TN, and TS; methodology, YT, and JF; software, YT; validation, JF, TS, AS, and TN; formal analysis, JF, and TN; investigation, YT; data curation, JF, and TN; writing—original draft preparation, JF, TS, YT and TN; writing—review and editing, JF, AS and TN; and; visualization and supervision, JF, AS and TN. All authors have read and agreed to the published version of the manuscript. All authors have contributed to the preparation of the manuscript and finally read and agreed to publish it in a journal.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sime, T., Fito, J., Nkambule, T.T.I. et al. Adsorption of Congo Red from Textile Wastewater Using Activated Carbon Developed from Corn Cobs: The Studies of Isotherms and Kinetics. Chemistry Africa 6, 667–682 (2023). https://doi.org/10.1007/s42250-022-00583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00583-2