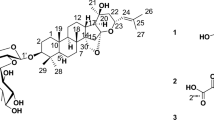
Abstract
In spite of remarkable progress and success made in anticancer research, major challenges in treatment of cancer remain recurrence of tumour and adverse effects of anticancer drugs. The search for novel, effective and safe anticancer agents, therefore remains important. This study investigated the chemical constituents of Phaulopsis falcisepala, a plant used in ethnomedicine to treat cancer; and evaluated anticancer and drug-likeness potentials of the constituents against selected non-small cell lung cancer (NSCLC), breast cancer, and chronic myelogenous leukemia (CML) targets. Lupeol and a mixture of stigmasterol and β-sitosterol were isolated from P. falcisepala and characterized using NMR, MS and IR data. Additional fifteen compounds, including piperine, piperanine, feruloyltyramine, asperglaucide and 6,7-dihydroflavopereirine were putatively identified in the plant by analysis of its MS/MS data against tandem MS/MS data repository using the Global Natural Products Social Molecular Networking tool (GNPS). Using molecular docking, stigmasterol (‒ 7.8 to ‒ 10.1 Kcal/mol), β-sitosterol (‒7.0 to ‒ 9.9 Kcal/mol), and lupeol (‒ 8.2 to ‒ 9.2 Kcal/mol) showed considerable binding affinities against non-small cell lung cancer and breast cancer molecular targets when compared to the reference inhibitors (‒ 8.4 to ‒ 10.8 Kcal/mol). Piperine (‒ 8.5 to ‒ 9.8 Kcal/mol), piperanine (‒ 8.5 to ‒ 9.6 Kcal/mol), and 6,7-dihydroflavopereirine (‒ 8.0 to ‒ 10.2 Kcal/mol) exerted good interactions with the active site, allosteric site and mutant form of BCR-ABL, an important target in chronic myelogenous leukemia. Many of the compounds in P. falcisepala were shown to possess fair to favourable drug likeness and ADME properties. However, lupeol, stigmasterol and β-sitosterol were predicted to have poor oral absorption.
Graphical Abstract








Similar content being viewed by others
References
World Health Organization (WHO) (2020) Global Health estimates 2020: deaths by cause, age, sex, by country and by region, 2000–2019. https://who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed 11 Dec 11 2020
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Olivier T, Haslam A, Prasad V (2021) Anticancer drugs approved by the US Food and Drug Administration from 2009 to 2020 according to their mechanism of action. JAMA Netw Open 4(12):e2138793. https://doi.org/10.1001/jamanetworkopen.2021.38793
Ali SM, Siddiqui R, Khan NA (2018) Antimicrobial discovery from natural and unusual sources. J Pharm Pharmacol 70(10):1287–1300. https://doi.org/10.1111/jphp.12976
El-Sayed KA (2000) Natural products as antiviral agents. In: ur-Rahman A (ed) Studies in natural products chemistry, vol 24. Part E. Elsevier, Amsterdam pp, pp 473–572
Oladipupo AR, Coker HAB, Alaribe CS, Okoko OD (2019) Microbial metabolites and enzymes inhibition in drug discovery and development: a case study of the Statins, a class of HMG-CoA reductase inhibitors. Trop J Nat Prod Res 3(4):99–106. https://doi.org/10.26538/tjnpr/v3i4.1
Alaribe CS, Oladipupo AR, Uche GC, Onumba MU, Awodele O, Oyibo WA (2021) Suppressive, curative, and prophylactic potentials of an antimalarial polyherbal mixture and its individual components in Plasmodium berghei-infected mice. J Ethnopharmacol 277:114105. https://doi.org/10.1016/j.jep.2021.114105
Mojab F (2012) Antimalarial natural products: a review. Avicenna J Phytomed 2(2):52–62
Oladipupo AR (2020) Toxin to medicine and bioisosterism in drug development: a study of the discovery and development of ACE inhibitors from snake venom. Maced Pharm Bull 66(2):15–33. https://doi.org/10.33320/maced.pharm.bull.2020.66.03.001
Calixto JB (2019) The role of natural products in modern drug discovery. An Acad Bras Cienc 91:e20190105. https://doi.org/10.1590/0001-3765201920190105
Qinghaosu Antimalarial Coordinating Research Group (1979) Antimalarial studies on Qinghaosu. Chin Med J (Engl) 12:811–816
Tu YY(1981) Fourth Meeting of the WHO Scientific Working Group on the Chemotherapy of Malaria: TDR/CHEMAL-SWG (4)/(QHS)/81.3; Beijing, China
Nobelprize.org (2015) The 2015 Nobel Prize in physiology or medicine-Press release. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/press.html. Accessed 17 Oct 2015
Burkill HM (1985) Entry for Phaulopsis falcisepala C.B.Cl. [family Acanthaceae]. In: Burkill HM (ed) The useful plants of West Tropical Africa, 2nd edn. Royal Botanical Garden, Kew, pp 388–389
Fongod AGN, Modjenpa NB, Veranso MC (2013) Ethnobotany of acanthaceae in the Mount Cameroon region. J Med Plants Res 7:2707–2713. https://doi.org/10.5897/JMPR11.1267
Oladipupo AR, Alaribe CS, Akintemi TA, Coker HAB (2021) Effect of Phaulopsis falcisepala (Acanthaceae) leaves and stems on mitotic arrest and induction of chromosomal changes in Meristematic cells of Allium cepa. Progress Chem Biochem Res 4(2):134–147. https://doi.org/10.22034/pcbr.2021.256993.1163
Abiodun OO, Tijani R, Ogbole O, Ajaiyeoba E (2018) Antioxidant, alpha-amylase and alpha-glucosidase inhibitory activities of leaf and flower extracts and fractions of Phaulopsis falcisepala C. B. Clarke. Acta Pharm Sci 56(4):23–33. https://doi.org/10.23893/1307-2080.APS.05623
Adesegun SA, Fajana A, Orabueze CI, Coker HAB (2009) Evaluation of antioxidant Properties of Phaulopsis falcisepala C.B.Cl. (Acanthaceae). Evid Based Comp Alt Med 6(2):227–231
Usman SO, Aliyu AA, Sowemimo AA, Sofidiya MO (2020) Anti-inflammatory investigations of the ethanol extract of Phaulopsis falcisepala C.B. Clarke (Acanthaceae) whole plant in rodents. Trop J Nat Prod Res 4(4):165–171. https://doi.org/10.26538/tjnpr/v4i4.8
Alaribe CS, Oladipupo AR, Ojo-Nosakhare O, Kehinde O, Ogunlaja AS (2020) GC-MS analysis and mitochondrial functionality potential of the fruits of Tetrapleura tetraptera by Cupric reducing antioxidant capacity assay. Phytomed Ther 19(1):338–347. https://doi.org/10.4314/jopat.v19i1.2
Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S et al (2012) A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30(10):918–920. https://doi.org/10.1038/nbt.2377
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504. https://doi.org/10.1101/gr.1239303
BIOVIA (2021) BIOVIA Discovery Studio Visualizer 21.1.0.0. Dassault Systèmes, San Diego
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
García GM, López CE, García NJ, Nebro A, Aldana MJ (2016) Molecular docking optimization in the context of multi-drug resistant and sensitive EGFR mutants. Molecules 21:1575. https://doi.org/10.3390/molecules21111575
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7(1):42717. https://doi.org/10.1038/srep42717
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45(12):2615–2623. https://doi.org/10.1021/jm020017n
Aniyery RB, Gupta A, Singh P, Sanju (2017) In-vitro and in silico antimicrobial study of stannane of pyridoxal 5-phosphate. Int J Pharm Pharm Sci 9(2):145–153. https://doi.org/10.22159/ijpps.2017v9i2.15002
Kumar NA, Sharmila R, Akila K, Jaikumar B (2016) In-silico approach for the assessment of oral cancer property on limonia acidissima. IJPSR 7(3):1271–1275
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13:714–726. https://doi.org/10.1038/nrc3599
Yardley DA (2013) Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer 2013:137414. https://doi.org/10.1155/2013/137414
Kengne ABO, Tene M, Tchinda AT, Tane P, Frédérich M (2016) Terpenoids from Phaulopsis imbricata (Acanthaceae). J Med Plants Res 10(10):122–129. https://doi.org/10.5897/JMPR2015.6027
Reher R, Kim HW, Zhang C, Mao HH (2020) A convolutional neural network-based approach for the rapid annotation of molecularly diverse natural products. J Am Chem Soc 142(9):4114–4120. https://doi.org/10.1021/jacs.9b13786
Mokgotho MP(2009) Isolation and characterisation of bioactive compounds from Commelina benghalensis Linn: biological activity analysis of extracts against Wil-2 NS lymphoma cancer cell lines and selected pathogenic microorganisms. PhD Thesis, University of Limpopo
Okoro IS, Tor-Anyiin TA, Igoli JO, Noundou XS, Krause RWM (2017) Isolation and characterisation of stigmasterol and β–Sitosterol from Anthocleista djalonensis A. Chev. Asian J Chem Sci 3(4):1–5. https://doi.org/10.9734/AJOCS/2017/37147
Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y et al (2016) Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular networking. Nat Biotechnol 34:828–837. https://doi.org/10.1038/nbt.3597
Ignacimuthu S, Shanmugam N (2010) Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from indian shrub Adhatoda vasica Ness. Leaves. J Biosci 35(4):565–570. https://doi.org/10.1007/s12038-010-0065-8
Abdel-Mageed WM, Fayed M, Al-Saleem M, Al-Wahaibi LH, Parvez MK, Li L, Al-Dosari MS, Sayed HM (2020) Novel polycyclic pyrroloquinazoline alkaloids from Anisotes trisulcus and their biological activity. J Asian Nat Prod Res 22(12):1159–1167. https://doi.org/10.1080/10286020.2019.1694514
Tiwari KP, Minocha PK, Masood M (1980) Acanthicifoline—a new alkaloid from Acanthus ilicifolius. Pol J Chem 54:857–858. https://doi.org/10.1002/chin.198048301
Minocha PK, Tiwari KP (1980) Chemical constituents of Acanthus ilicifolius Linn. Pol J Chem 54:2089–2090
Murty MSR, Kamat SYS (1984) Isolation of 2-benzoxazolinone from Acanthus ilicifolius. Indian J Pharm Sci 46:218–219
Kokpol U, Chittawong V, Miles DH (1986) Chemical constituents of the roots of Acanthus ilicifolius. J Nat Prod 49:354. https://doi.org/10.1021/np50044a033
Hedberg C, Hesse M, Werner C (1996) Spermine and spermidine hydroxyl cinnamoyl transeferases in Aphelandra tetragona. Plant Sci 113:149–156
Calderón AI, Hodel A, Wolfender JL, Gupta MP, Correa M, Hostettmann K (2013) LC-DAD-MS-based metabolite profiling of three species of Justicia (Acanthaceae). Nat Prod Res 27(15):1335–1342. https://doi.org/10.1080/14786419.2012.738207
Petit-Topin I, Fay M, Resche-Rigon M, Ulmann A, Gainer E, Rafestin-Oblin ME, Fagart J (2014) Molecular determinants of the recognition of ulipristal acetate by oxo-steroid receptors. J Steroid Biochem Mol Biol 144:427–435. https://doi.org/10.1016/j.jsbmb.2014.08.008
Imberty A, Hardman KD, Carver JP, Perez S (1991) Molecular modelling of protein-carbohydrate interactions. Docking of monosaccharides in the binding site of concanavalin A. Glycobiology 1:631–642. https://doi.org/10.1093/glycob/1.6.631
Sali VK, Mansingh DP, Vasanthi HR (2016) Relative apoptotic potential and specific G1 arrest of stigmasterol and cinnamic acid isolated from the brown algae Padina gymnospora in HeLa and A549 cells. MedChemComm 7:1429–1435. https://doi.org/10.1039/c6md00178e
Shen T, Zhang L, Wang YY, Fan PH, Wang XN, Lin ZM, Lou HX (2012) Steroids from Commiphora mukul display antiproliferative effect against human prostate cancer PC3 cells via induction of apoptosis. Bioorg Med Chem Lett 22(14):4801–4806. https://doi.org/10.1016/j.bmcl.2012.05.052
Ali H, Dixit S, Ali D, Alqahtani SM, Alkahtani S, Alarifi S (2015) Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des Devel Ther 9:2793–2800. https://doi.org/10.2147/dddt.s83514
AmeliMojarad M, AmeliMojarad M, Pourmahdian A (2022) The inhibitory role of stigmasterol on tumor growth by inducing apoptosis in Balb/c mouse with spontaneous breast tumor (SMMT). BMC Pharmacol Toxicol. https://doi.org/10.1186/s40360-022-00578-2
Borner MM, Brousset P, Pfanner-Meyer B, Bacchi M, Vonlanthen S, Hotz MA, Altermatt HJ, Schlaifer D, Reed JC, Betticher DC (1999) Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non small-cell lung cancer. Br J Cancer 79:952–958. https://doi.org/10.1038/sj.bjc.6690152
Kim YS, Li XF, Kang KH, Ryu BM, Kim SK (2014) Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep 47:433–438. https://doi.org/10.5483/bmbrep.2014.47.8.153
Zhao H, Zhang X, Wang M, Lin Y, Zhou S (2021) Stigmasterol simultaneously induces apoptosis and protective autophagy by inhibiting Akt/mTOR pathway in gastric Cancer cells. Front Oncol 11:43. https://doi.org/10.3389/fonc.2021.629008
Ding Y, Nguyen HT, Kim SI, Kim HW, Kim YH (2009) The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorg Med Chem Lett 19(13):3607–3610. https://doi.org/10.1016/j.bmcl.2009.04.129
Rajavel T, Mohankumar R, Archunan G, Ruckmani K, Devi KP (2017) Beta sitosterol and daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep 7(1):3418. https://doi.org/10.1038/s41598-017-03511-4
Moon DO, Kim MO, Choi YH, Kim GY (2008) beta-sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett 264(2):181–191. https://doi.org/10.1016/j.canlet.2008.01.032
Zhou J, Li CJ, Yang JZ, Ma J, Li Y, Bao XQ, Chen XG, Zhang D, Zhang DM (2014) Lupane triterpenoids from the stems of Euonymus carnosus. J Nat Prod 77(2):276–284. https://doi.org/10.1021/np400851k
Taleghani A, Emami SA, Tayarani-Najaran Z (2020) Artemisia: a promising plant for the treatment of cancer. Bioorg Med Chem 28(1):115180. https://doi.org/10.1016/j.bmc.2019.115180
Lin LC, Chou CJ, Kuo YC (2001) Cytotoxic principles from Ventilago leiocarpa. J Nat Prod 64(5):674–676. https://doi.org/10.1021/np000569d
Umadevi P, Deepti K, Venugopal DVR (2013) Synthesis, anticancer and antibacterial activities of piperine analogs. Med Chem Res 22(11):5466–5471. https://doi.org/10.1007/s00044-013-0541-4
Li N, Wen S, Chen G, Wang S (2020) Antiproliferative potential of piperine and curcumin in drug-resistant human leukemia cancer cells are mediated via autophagy and apoptosis induction, S-phase cell cycle arrest and inhibition of cell invasion and migration. J BUON 25(1):401–406
Lin Y, Xu J, Liao H, Li L, Pan L (2014) Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol 35(4):3305–3310. https://doi.org/10.1007/s13277-013-1433-4
Greenshields AL, Doucette CD, Sutton KM, Madera L, Annan H, Yaffe PB, Knickle AF, Dong Z, Hoskin DW (2015) Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett 357(1):129–140. https://doi.org/10.1016/j.canlet.2014.11.017
Xia Y, Khoi PN, Yoon HJ, Lian S, Joo YE, Chay KO, Kim KK, Jung YD (2015) Piperine inhibits IL-1β-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol Cell Biochem 398(1–2):147–156. https://doi.org/10.1007/s11010-014-2214-0
Si L, Yang R, Lin R, Yang S (2018) Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci Rep 38(3):BSR20180503. https://doi.org/10.1042/BSR20180503
Mitra S, Anand U, Jha NK, Shekhawat MS, Saha SC, Nongdam P, Rengasamy KRR, Proćków J, Dey A (2022) Anticancer Applications and Pharmacological Properties of Piperidine and Piperine: a Comprehensive Review on Molecular Mechanisms and therapeutic perspectives. Front Pharmacol 12:772418. https://doi.org/10.3389/fphar.2021.772418
Hamidović A, Hasković E, Muhić S, Planinić M, Omerović N, Škrbo S(2021) Application of in silico methods in pharmacokinetic studies during drug development. In: Badnjevic A Gurbeta Pokvić L (eds) CMBEBIH 2021, IFMBE Proceedings 84:499–510. https://doi.org/10.1007/978-3-030-73909-6_58
Valerio LG Jr (2012) Application of advanced in silico methods for predictive modeling and information integration. Expert Opin Drug Metab Toxicol 8(4):395–398. https://doi.org/10.1517/17425255.2012.664636
Yamashita F, Hashida M (2004) In silico approaches for predicting ADME properties of drugs. Drug Metab Pharmacokinet 19(5):327–338. https://doi.org/10.2133/dmpk.19.327
Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR (2015) Molecular determinants of blood–brain barrier permeation. Thera Deliv 6:961–971. https://doi.org/10.4155/tde.15.32
Testa B, Kraemer SD (2007) The Biochemistry of Drug metabolism – an introduction. Chem Biodivers 4(3):257–405. https://doi.org/10.1002/cbdv.200790032
Hollenberg PF (2002) Characteristics and common properties of inhibitors, inducers, and activators of CYP enzymes. Drug Metab Rev 34:17–35. https://doi.org/10.1081/dmr-120001387
Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R et al (2008) New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol 48:662–670. https://doi.org/10.1177/0091270007312153
Baell JB, Holloway GA (2010) New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem 53:2719–2740. https://doi.org/10.1021/jm901137j
Brenk R, Schipani A, James D, Krasowski A (2008) Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 3:435–444. https://doi.org/10.1002/cmdc.200700139
Acknowledgements
The authors appreciate the Department of Chemistry, Nelson Mandela University for the provision of facilities for isolating and elucidating the structures of compounds and the Département de Pharmacognosie, Faculté de Pharmacie, Université Paris-Saclay for the facilities for the UPLC-HRMS/MS analysis.
Funding
This study was supported by Tertiary Education Trust Fund (TETFUND) under Grant: DR&D/CE/NRF/STI/70/VOL.I and Royal Society of Chemistry (RSC) under Grant: R20-4483.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oladipupo, A.R., Alaribe, S.C.A., Ogunlaja, A.S. et al. Chemical and Biological Insights on Phaulopsis falcisepala: A Source of Bioactive Compounds with Multifunctional Anticancer Potentials. Chemistry Africa 6, 1175–1189 (2023). https://doi.org/10.1007/s42250-022-00553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00553-8