Abstract
This study delves into the synthesis of CaSO\(_{4}\) materials using the Pechini and sol-gel methods, followed by heat treatment up to 900 \(^{\circ }\)C. X-ray analysis discerned distinct phases, with one method yielding pure orthorhombic anhydrite and the other predominantly producing a mixture of orthorhombic anhydrite and bassanite. Scanning electron microscopy (SEM) showcased homogeneous columnar morphologies for the anhydrite samples and irregularly sized particles for the alternative method. Photoluminescence (PL) measurements demonstrated emissions in the visible range (approximately 400 nm to 700 nm) with differences noted between synthesis methods. Low-temperature Raman spectroscopy (20 K to room temperature) unveiled unique vibrational modes, notably with the orthorhombic phase of anhydrite exhibiting the most intense peak at 1017 cm\(^{-1}\). These powders, intended for thin film applications, bear significance in electronics, optoelectronics, and nanotechnology. A thorough comprehension of their structural and vibrational properties at the microscopic level is paramount for material and device design and optimization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, significant advancements have been made in the synthesis of calcium oxides, leading to the production of these compounds in various phases such as cubic (CaO) and hexagonal (CaO). Additionally, diverse morphologies, including nanoparticles, nanowires or nanotubes, and porous nanostructures, have been achieved. Each phase and morphology is characterized by distinct properties, opening up a spectrum of potential applications. The exploration of different phases of calcium oxides has emerged as a focal point in materials science, spurred by the pursuit of tailored functionalities to address the evolving demands of modern technologies [1,2,3,4,5]. This emphasis on the different phases of calcium oxides is particularly pertinent in the field of bioceramics, where calcium and its compounds, such as calcium sulfate (gypsum), have found extensive application. Their significant role is especially notable in the advancement of bone graft substitutes for fracture repair [1, 6]. Bioceramics are typically categorized as either resorbable or non-resorbable materials. Calcium sulfate (CaSO\(_{4}\)) powder has been widely employed as a filler for bone fractures. However, in order to utilize it as a bone graft substitute, it is crucial to enhance the resistance of CaSO\(_{4}\) and reduce its degradation rate. This necessitates improving the densification during the synthesis process and conducting further research on the biodegradation and cytotoxicity of this material [1, 7,8,9].
This research is closely aligned with extensive studies on wollastonite (CaSiO\(_{3}\)) within scholarly literature, aimed at mitigating the risk of post-surgical bone infections. Specifically, researchers have focused on exploring the potential of antibacterial scaffolds incorporating wollastonite to prevent such infections. Moreover, these studies have comprehensively examined the applications of calcium silicate (CaSiO\(_{3}\)) as a biocompatible material, contributing to the restoration of robust hard tissues [3, 4]. Notably, the remarkable bioactivity of CaSiO\(_{3}\), surpassing that of hydroxyapatite [3], has garnered significant interest in its use for bone tissue regeneration applications. With its cost-effectiveness and exceptional biomechanical properties, CaSiO\(_{3}\) emerges as a promising candidate in this field [4, 10].
Building upon the advancements in the exploration of wollastonite, which holds significant promise for applications in bone health, the scientific community has concurrently directed attention towards addressing broader environmental concerns. This has led to a burgeoning interest in mitigating CO\(_{2}\) emissions by developing composites that offer distinctive advantages over conventional materials [5, 11]. Within this environmental context, the production of calcium carbonate emerges as a particularly promising avenue for carbon capture and utilization technologies [5].
The successful implementation of calcium carbonate in carbon capture and utilization technologies hinges upon precise control over its growth and morphology. While the controlled synthesis of nanoscale calcium carbonate (CaCO\(_{3}\)) particles offers promising prospects for drug delivery systems due to their biocompatibility, biodegradability, cost-effectiveness, simplicity, and stimulus-responsiveness, it also addresses the challenge of achieving precise control over particle size in the nanometer range through scalable manufacturing methods. At the nanometer level, calcium carbonate particles exhibit a high surface area-to-volume ratio and enhanced reactivity, enabling efficient encapsulation and controlled release of drugs, thereby facilitating targeted delivery to specific tissues or cells. Furthermore, their ability to bypass biological barriers more effectively enhances bioavailability and therapeutic efficacy. Hence, the controlled synthesis of nanoparticles is imperative for optimizing drug delivery systems and augmenting their therapeutic outcomes [5].
The significance of material synthesis methods in obtaining materials with controlled, optimized, and reproducible properties cannot be overstated. These methods play a crucial role in driving scientific progress, fostering technological innovation, and facilitating the development of new products and applications [12,13,14,15,16,17]. Consequently, in this study, two distinct synthesis routes were employed to compare the obtained results and identify potential candidate materials for specific applications based on their achieved properties. Raman spectroscopy is a highly sensitive technique commonly employed for the characterization of such systems, enabling the differentiation of polymorphic compounds and calcium oxides. However, inconsistencies in the literature regarding the definition of each phase exist, particularly concerning bassanite and anhydrite phases, as highlighted by Prieto-Taboada et al. [18].
In this study, we investigated the influence of annealing on the samples using two distinct synthesis methods (Pechini and sol-gel). The main focus of our research was to analyze the morphology of the samples obtained from these methods, considering the absence of pure phases in both cases. Additionally, photoluminescence measurements revealed distinct emission patterns for each synthesis method. Furthermore, Raman spectroscopy was employed to study the vibrational modes across a temperature range from 20 K to room temperature, providing insights into their dynamics.
2 Materials and methods
For route (1) the powder was obtained by the Pechini method. Initially, 10.0305 ml of ethylene glycol (Panreac 99.8%) were heated to 70 \(^{\circ }\)C and 9.4559 g of monohydrated citric acid (Panreac 99.8%) was slowly added, keeping the system temperature constant at 70 \(^{\circ }\)C, and continuously stirring the mixture, until getting a transparent solution. Separately, the precursor solution was prepared at 0.3M (2.5356g) of calcium sulfate dihydrate (CaSO\(_{4}\cdot 2\)H\(_{2}\)O-Merck 98.2%) in an aqueous solution of 0.4M nitric acid (HNO\(_{3}\) Panreac 65%). The calcium precursor solution was added to the mixture of ethylene glycol and citric acid and when it became semitransparent the temperature was reduced to 25 \(^{\circ }\)C to start adding NH\(_{4}\)OH (Panreac 30%) until the solution reached a pH of 5. Ensuring that no precipitate had formed in the now clear system, the solution was heated to 90 \(^{\circ }\)C, with constant stirring, until a white resin had formed. The resin obtained was pre-calcined at 30 \(^{\circ }\)C and the solid resulting from this pre-calcination was macerated using an agate mortar. The obtained powders were subjected to final heat treatments from 300 \(^{\circ }\)C to 900 \(^{\circ }\)C [19].
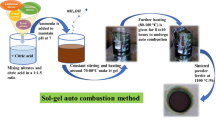
Sol-Gel Synthesis Method Description: Precursor Solution Preparation: Disperse 40.47 g of calcium methoxide merck in 500 mL of anhydrous ethanol, supplemented with the addition of 100 mL of water. Adjustment of pH and Catalysis: Set the pH to 3 using HCl or HNO\(_{3}\) as a hydrolysis catalyst. Gelation Step: The mixture was refluxed with constant stirring at 80 \(^{\circ }\)C for 24 h until gelation occurred. The gel was subsequently subjected to a drying process at 100 \(^{\circ }\)C. Thermal Treatment: Finally, the sample underwent thermal treatment across a temperature range from 300 \(^{\circ }\)C to 900 \(^{\circ }\)C.
The structural analysis of the compounds was performed through X-ray diffraction. The equipment used was Shimadzu XRD-6000, operating with Cu-K\(\alpha \) radiation, \(\lambda \) 1.5406 Å at 1\(^{\circ }\)C min\(^-1\) from 2\(\theta \) 5\(^{\circ }\) to 80\(^{\circ }\). The images were obtained via scanning electron microscopy (SEM) equipment, JEOL model 6701F, using at 5 kV. The PL measurements were performed with a monospec 27 monochromator (Thermal Jarrel AS, USA) coupled to an R446 photomultiplier (Hamamatsu photonics, Japan). A Krypton ion laser (Coherent Innova 90 K, USA) (\(\lambda \)=350 nm) was used as excitation source. Raman scattering spectra were obtained in the backscattering geometry with a Jobin-Yvon-T64000 spectrometer, coupled to a N\(_{2}\) cooled CCD device, using as excitation the 532 nm laser line of a Verdi laser, operating at a low power. For measurements over a wide range of temperatures an Air Products closed-cycle system was employed.
3 Results
SEM measurements play a crucial role in characterizing CaSO\(_{4}\) samples as they provide valuable insights into the morphology and surface structure of the synthesized particles. These measurements allow for an evaluation of particle size uniformity. In Fig. 1, the SEM images depict the results obtained from the first synthesis method. Figure 1(a) shows SEM images of the sample of the material pre-calcined in the synthesis by method 1. The material is observed agglomerated without any particular defined shape, the guide bar is 2 \(\mu \)m. In (b) the image of the material at 650 \(^{\circ }\)C and a 100-nm guide. The particles are not homogeneous, different particle sizes are observed that vary approximately between 100 nm and 200 nm. In (c) the material at 800 \(^{\circ }\)C and its respective 100 nm guide shows some plate-like shapes; however, they are not uniform particles and finally in (d) the material at 900 \(^{\circ }\)C with larger particle sizes and embedded with each other, block-like. This morphology is similar to that found by Satoru [20] in calcite. As can be seen in Fig. 1, the morphology is not uniform and the particle size increases with the annealing temperature.
Figure 2 shows the samples for method 2: (a) the pre-calcined sample is depicted, and the guide bar is 10 \(\mu \)m. No definite structure is observed in this figure. (b) Column-like structures with varying sizes, both large and small, are observed. This measurement corresponds to the sample calcined at 650 \(^{\circ }\)C. (c, d) These images display the sample calcined at 800 \(^{\circ }\)C using a 1-\(\mu \)m guide. The columnar morphology is well-defined and uniform, with the formed columns measuring over 4 \(\mu \)m in length. (e, f) The sample subjected to calcination at 900 \(^{\circ }\)C is shown. It appears fractured, with some columns exhibiting a larger diameter but shorter length compared to the 800 \(^{\circ }\)C temperature. Furthermore, the morphology obtained in this work exhibits a similar resemblance to that reported by Tomi et al. [21] in the literature, with the key difference being that the morphology observed in our samples is more homogeneous. This suggests that the uniform columnar morphologies may be disrupted after this calcination temperature. These findings suggest that the calcination temperature significantly influences the morphology and organization of the CaSO\(_{4}\) samples.
In Table 1, a comparison is drawn between the grain sizes of pre-calcined material in method 1 and method 2, emphasizing noteworthy differences in the resulting morphologies. In method 1, agglomerations are noted, lacking a distinct definition in particle morphology. On the other hand, method 2 reveals the onset of irregular rectangular bar morphologies and clusters, each exhibiting lengths of approximately 5.3 micrometers. These dimensions were calculated utilizing the ImageJ open-source software. Upon examining the data at various calcination temperatures, a trend becomes evident, indicating an increase in both width and length dimensions as the calcination temperature rises for both methods. The sizes are notably larger for method 2, displaying a morphology clearly in the form of rectangular bars. In contrast, for method 1, the sizes are significantly smaller, exhibiting irregular morphologies resembling small rectangles. It is noteworthy to highlight that for method 2, the size at 650 \(^{\circ }\)C is considerably larger compared to 900 \(^{\circ }\)C. However, it is observed to be quite fragile, forming several agglomerated rectangular bars. It is essential to recognize that the outcomes are sensitive to both the calcination temperature and the synthesis method. These factors have the potential to modify the physicochemical properties, introducing variations that may impact the material’s suitability for diverse applications.
Figure 3 displays the x-ray measurements of the samples from method 1 that underwent calcination temperatures ranging from 450 \(^{\circ }\)C to 650 \(^{\circ }\)C. In these measurements, the presence of the Anhydrite Orthorhombic phase is evident [1], accompanied by the monoclinic phase [22, 23]. As can be observed in Fig. 3, the indexed peaks correspond to the anhydrite phase (PDF#37 1496). In this context, distinguishing the X-ray peaks from the bassanite phase proves challenging as they overlap with each other, as well as those of the CaSO\(_{4}\).2H\(_{2}\)O phase [24, 25].
X-ray measurements indicate that the prevailing phase within the sample is the orthorhombic phase of anhydrite, making up a substantial portion in a semi-quantitative analysis. Additionally, there is an observation of a secondary hexagonal phase, contributing to a significant part of the overall composition [22]. The intensity of the peaks associated with the crystallographic planes of the less predominant phase is noteworthy, especially in comparison to the intensity of the peaks observed in the x-ray measurements using method 2, as illustrated in Fig. 4.
These findings highlight the varied phases achieved through several synthesis methods. The contrasting peak intensities associated with the minority phase in both method 1 and method 2 underscore the significant influence of synthesis approaches on the resulting phase. Figure 4 illustrates the results of method 2 across a temperature range, from pre-calcination up to \(650^{\circ }\)C. The pre-calcined material exhibits less defined peaks. However, from 350 \(^{\circ }\)C to 650 \(^{\circ }\)C, X-ray diffractograms show narrower and more defined peaks, suggesting an increase in material crystallinity.
An observation can be made regarding the alteration in maximum intensity as the calcination temperature increases, indicating an enhancement in crystallinity. Particularly, the diffractograms suggest that the primary phase is predominantly CaSO\(_{4}\) or anhydrite. This implies that synthesis method 2 is more effective in attaining the pure anhydrite phase.
The XRD patterns depicted in Fig. 5 for method 2 exhibit the presence of a single-phase orthorhombic anhydrite (PDF \( \# 96-\!900-\!4097\)) in the sintered samples. The values provided in the format of 2\(\theta \) (hkl) represent the Miller indices of the crystalline planes in the corresponding 2\(\theta \) positions. Notably, the following peaks were observed: 22.9 (111), 25.4 (002), 28.6 (020), 31.4 (021), 31.9 (112), 36.4 (202), 38.6 (022), 40.8 (221), 41.4 (310), 43.4 (113), 45.4 (130), 46.8 (222), 48.7 (131), 49.1 (312), 52.2 (400), 55.7 (223), 57.8 (313), 59.0 (402), 60.6 (420), 61.0 (331), 62.2 (421), 65.4 (240), 65.7 (332), 66.9 (241), 68.7 (314), 71.4 (242), 72.8 (043), 74.2 (423), 74.3 (025), 77.1 (404), 78.6 (243), 79.8 (225). This indicates the presence of a pure phase in the sample, specifically pure calcium sulfate (CaSO\(_{4}\)), which holds significance for its application in bone regeneration grafts [1].
In method 1 of Fig. 5, where a calcination temperature of 900 \(^{\circ }\)C was applied, an additional phase, namely CaSO\(_{4}\).(H\(_{2}\)O), was observed. However, it should be noted that this phase is not pure, as indicated by the presence of peaks in the 2\(\theta \) range corresponding to the crystallographic plane (hkl) of the hexagonal phase CaSO\(_{4}\)(H\(_{2}\)O).\(_{8}\), with code 96-202-0134. Specifically, peaks were observed at 2\(\theta \) values of 29.5 (200), 35.5 (10-2), and 48.8 (212). However, it is important to highlight that this phase is present in a minority quantity. These findings suggest that the desired pure phase cannot be obtained at 900 \(^{\circ }\)C using this particular synthesis method and that a longer calcination time may be required to achieve the desired pure phase (CaSO\(_{4}\)). The other peaks correspond to the orthorhombic anhydrite phase. Stawski et al. [26] assert that the formation process of gypsum is not fully understood under traditional models of nucleation and crystal growth, prompting extensive research to better grasp these mechanisms. It is posited that gypsum exhibits a mesocrystalline nature, comprising crystalline domains “glued” together by disordered groups, enabling a significant proportion of these groups to coexist within the solid alongside the crystalline gypsum. Optimizing this compound is paramount given literature reports [27] suggesting its versatility due to complete reabsorption within a short timeframe. Additionally, it exhibits high biocompatibility, serving as a resorbable scaffold for bone growth, supplying calcium ions to stimulate osteoblastic activity, and enabling the administration of growth factors, small molecule drugs, and antibiotics. However, synthesis and optimization remain subjects of ongoing study.
Figure 6(a) displays the photoluminescence spectra of the CaSO\(_{4}\) sample synthesized using method 1 and calcined at 650 \(^{\circ }\)C. The dominant emissions are observed at 456 nm and 579 nm [17]. Conversely, in Fig. 6(b) for the same synthesis method but at 900 \(^{\circ }\)C, the main emissions occur at 449 nm and 587 nm. Additionally, the peaks in this calcination condition are narrower, indicating higher crystallinity. Moving on to Fig. 6(c), the sample synthesized using method 2 at 650 \(^{\circ }\)C exhibits a maximum peak and main emission at 586 nm. However, in Fig. 6(d), the sample at 900 \(^{\circ }\)C using method 2 shows narrower peaks compared to the sample at the same temperature using the previous method. The main emissions for this sample are at 601 nm and 453 nm, corresponding to energy levels of approximately 2.01 eV and 2.1 eV, respectively. Observing the field of photoluminescence, these materials could be applied in the manufacturing of optoelectronic devices. The wavelengths and energies observed align with those of certain semiconductor materials, suggesting that these samples may possess intriguing semiconductor properties for light detection applications such as photodetectors. Band gap similar to Group III-V Based 2D Materials [28]. The variation in the wavelength of the emission peaks may be attributed to differences in both structure and particle size among the samples. In method 1, there is a smaller particle size, whereas in method 2, there is a larger size [29].
Furthermore, Raman measurements were conducted on the samples synthesized using both methods, covering the temperature range from 20 K down to room temperature. Figure 7(a) illustrates the Raman spectra obtained from the sample annealed at 900 \(^{\circ }\)C using method 1. The Raman measurements were carried out using a cryostat, with the temperature varying from 20 K to 300 K. In Fig. 7(a), the Raman spectrum of method 1 is displayed, exhibiting vibrations at 417 cm\(^{-1}\) and 500 cm\(^{-1}\), which are associated with the \(\vartheta \)2 mode of the (SO\(_{4}\) ) group. These vibrations show a higher intensity at 20 K and gradually increase as the temperature approaches room temperature [18]. The vibrational modes located at 610 cm\(^{-1}\), 627 cm\(^{-1}\), and 674 cm\(^{-1}\) correspond to the \(\vartheta \)4 modes of (SO\(_{4}\) ). Additionally, the intense and characteristic \(\vartheta \)1 mode of (SO\(_{4}\) ) is observed at 1018 cm\(^{-1}\), indicating the presence of the anhydrite II phase [18, 30]. The \(\vartheta \)3 mode of (SO\(_{4}\) ) is also detected at 1113 cm\(^{-1}\), 1130 cm\(^{-1}\), and 1162 cm\(^{-1}\)1. These vibrational modes belong to the dominant anhydrite II phase, but there are two additional peaks at 484 cm\(^{-1}\) and 1088 cm\(^{-1}\) that are not reported in the literature and may be associated with a minority phase in the sample. It is worth noting that the relative intensity of the Raman vibrational modes decreases with increasing temperature across all spectra. These results, based on the peak positions of Raman vibrational modes, corroborate the presence of the anhydrite and bassanite phases, as observed in the X-ray measurements [30]. The vibrational modes between 1050 cm\(^{-1}\) and 1200 cm\(^{-1}\) appear to correspond to the active Raman modes of the bassanite phase. Figure 7(b) displays the Raman spectra of the sample synthesized using method 2 and calcined at 900 \(^{\circ }\)C. Similar to Fig. 7(a), Raman measurements were conducted from 20 K to room temperature. In this case, the observed vibrational modes correspond to the anhydrite II phase, and no additional vibrational modes beyond those characteristic of this phase are observed. This suggests that the phase obtained is pure and free from secondary phases. Notably, Fig. 7(b) displays higher intensity across all vibrational modes and at different temperatures compared to the spectra of the sample shown in Fig. 7(a).
Figure 8 presents the Raman spectra obtained for method 2 at room temperature, displaying the samples calcined at 650 \(^{\circ }\)C, 700 \(^{\circ }\)C, 800 \(^{\circ }\)C, and 900 \(^{\circ }\)C. Notably, for samples calcined below 900 \(^{\circ }\)C, a broad luminescence band is observed. However, for the 900 \(^{\circ }\)C sample, this broad band is absent, and the baseline appears almost straight. In all spectra, regardless of the temperature, only vibrational modes corresponding to the anhydrite II phase are present [18, 22]. It is worth noting that the intensity of the peaks is lower and the peak lengths are slightly longer for the samples calcined at lower temperatures compared to the 900 \(^{\circ }\)C sample. In this case, it seems that no other phase besides anhydrite is present.
4 Conclusions
The Pechini and sol-gel synthesis methods have proven to be efficient and cost-effective means of obtaining CaSO\(_{4}\) samples. X-ray diffraction measurements revealed varying proportions of anhydrite and bassanite phases, depending on the synthesis method and calcination temperature, with sol-gel emerging as the preferred method for obtaining the pure phase. This highlights the substantial influence of synthesis method and calcination temperature on the resultant phase obtained. SEM analysis revealed distinctive morphologies: method 2 exhibited a uniform columnar structure, while method 1 displayed irregular particle shapes but with smaller sizes. Photoluminescence (PL) measurements provided valuable insights into the optical properties of the CaSO\(_{4}\) samples within the semiconductor range, with emission energies around 2.01 eV and 2.1 eV. Low-temperature Raman spectroscopy identified vibration modes specific to the pure orthorhombic anhydrite phase in method 2 samples, whereas method 1 samples exhibited vibration modes associated with two phases. These Raman measurements further clarified the structural characteristics of the CaSO\(_{4}\) samples. Overall, these findings enhance our understanding of CaSO\(_{4}\) properties and offer pertinent insights for its potential applications across various fields, including materials science, medicine, and catalysis.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
S.T. Kuo, H.W. Wu, W.H. Tuan, Resorbable calcium sulfates with tunable degradation rate. J. Asian Ceram. Soc. 1, 102–107 (2013). https://doi.org/10.1016/j.jascer.2013.03.007
M. Mehrabi, M. Zahedifar, E. Sadeghi, Luminescence Properties Of Pure CaSO\(_{4}\) Nanoparticles Produced By Co-Precipitation Method. J. Nanostruct. 4, 425 (2014). https://doi.org/10.7508/jns.2014.04.004
K. Pawan, S.D. Brijnandan, S. Anil, K. Ravinder, I.P. Catalin, Y. Anil, Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 195, 109026 (2020). https://doi.org/10.1016/j.matdes.2020.109026
Y. Pan, J. Yin, D. Yao, K. Zuo, Y. Xia, H. Liang, Y. Zeng, Effects of silica sol on the microstructure and mechanical properties of CaSiO\(_{3}\) bioceramics. Mater. Sci. Eng. C 64, 336–340 (2016). https://doi.org/10.1016/j.msec.2016.03.109
J. Gu, T. Wang, G. Fan et al., Biocompatibility of artificial bone based on vancomycin loaded mesoporous silica nanoparticles and calcium sulfate composites. J. Mater. Sci.: Mater. Med. 27, 64 (2016). https://doi.org/10.1007/s10856-016-5671-z
Y.H. Chiu, I.C. Chen, C.Y. Su, H.H. Tsai, T.H. Young, H.W. Fang, Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. Int. J. Mol. Sci. 23, 7590 (2022). https://doi.org/10.3390/ijms23147590
N.T.N. Le, N.T.T. Le, Q.L. Nguyen, T.L. Pham, M.T. Le Nguyen, D.H. Nguyen, A Facile Synthesis Process and Evaluations of \(\alpha \)-Calcium Sulfate Hemihydrate for Bone Substitute. Materials (Basel) 13(14), 3099 (2020). https://doi.org/10.3390/ma13143099
C.M. Kelly, R.M. Wilkins, S. Gitelis, C. Hartjen, J.T. Watson, P.T. Kim, The Use of a Surgical Grade Calcium Sulfate as a Bone Graft Substitute. Clin. Orthop. Relat. Res. 382, 42–50 (2001). https://doi.org/10.1097/00003086-200101000-00008
Ching-Chien. Chiang et al., Cytotoxicity and cell response of preosteoblast in calcium sulfate-augmented PMMA bone cement. Biomed. Mater. 16, 055014 (2021). https://doi.org/10.1088/1748-605X/ac1ab5
P. Marco, C. Cédric, M. Lorenzo, A. Erwan, O. Roberto, D.J. Roberto, The Raman spectrum of CaCO\(_{3}\) polymorphs calcite and aragonite: A combined experimental and computational study. J. Chem. Phys. 140, 164509 (2014). https://doi.org/10.1063/1.4871900
A.C.F. Prado, J.O.D. Malafatti, J.A. Oliveira, C. Ribeiro, M.R. Joya, A.P. Luz, E.C. Paris, Preparation and Application of Nb\(_{2}\)O\(_{5}\) Nanofibers in CO\(_{2}\) Photoconversion. Nanomaterials 11(12), 3268 (2021). https://doi.org/10.3390/nano11123268
M.R. Joya, J.B. Ortega, J.O.D. Malafatti, E.C. Paris, Evaluation of Photocatalytic Activity in Water Pollutants and Cytotoxic Response of \(\alpha \)-Fe\(_{2}\)O\(_{3}\) Nanoparticles. ACS Omega 4(17), 17477–17486 (2019). https://doi.org/10.1021/acsomega.9b02251
L.J. Cardenas-Flechas, A.M. Raba, M.R. Joya, Synthesis and evaluation of nickel doped Co\({3}\)O\({4}\) produced through hydrothermal technique. Dyna 87, 184–191 (2020)
A.M. Raba, M.R. Joya, J.H. Bautista Ruiz, Quicklime as an alternative in the photodegradation of contaminants. J. Phys. Conf. Ser. 687, 012044 (2016). https://doi.org/10.1088/1742-6596/687/1/012044
M.R. Joya, J. Barba-Ortega, E.C. Paris, Obtención de muestras de óxidos a bajo costo. Rev. UIS Ing. 18(3), 33–37 (2019). https://doi.org/10.18273/revuin.v18n3-2019003
F. Persano, C. Nobile, C. Piccirillo, G. Gigli, S. Leporatti, Monodisperse and Nanometric-Sized Calcium Carbonate Particles Synthesis Optimization. Nanomaterials 12(9), 1494 (2022). https://doi.org/10.3390/nano1209149
M. Mohsen, Z. Mostafa, S. Ehsan, Luminescence Properties Of Pure CaSO\(_{4}\) Nanoparticles Produced By CoPrecipitation Method. JNS 4, 425–431 (2014). https://doi.org/10.7508/JNS.2014.04.004
N. Prieto-Taboada, O. Gómez-Laserna, I. Martínez-Arkarazo, M.Á. Olazabal, J.M. Madariaga, Raman Spectra of the Different Phases in the CaSO\(_{4}\)-H\(_{2}\)O System. Anal. Chem. 86(20), 10131–10137 (2014). https://doi.org/10.1021/ac501932f
M.R. Joya, A.M. Raba, J. Barba-Ortega, Synthesis of calcium oxide by means of two different chemical processes. Univ. Ciencia Tecnol. 20(81), 188–192 (2016)
F. Satoru, One-Step Synthesis of Calcium Sulfate Hemihydrate Nanofibers from Calcite at Room Temperature. ACS Omega 3(3), 2820–2824 (2018). https://doi.org/10.1021/acsomega.7b01994
G. Tomi, L. Andrej, P. Ciril, Continuous precipitation of calcium sulfate dihydrate from waste sulfuric acid and lime. J. Chem. Technol. Biotechnol. 80, 939–947 (2005). https://doi.org/10.1002/jctb.1266
S. Thomas, J. Robert, D. Petra, Insights into the CaSO\(_4\)-H\(_2\)O System: A Raman-Spectroscopic Study. Minerals 10(2), 115 (2020). https://doi.org/10.3390/min10020115
F. Beaugnon, S. Quiligotti, S. Chevreux, G. Wallez, On the monoclinic distortion of \(\beta \)-anhydrite CaSO\(_{4}\). Solid State Sci. 108, 106399 (2020). https://doi.org/10.1016/j.solidstatesciences
Shu-Ting. Kuo, Hao-Wei. Wu, Wei-Hsing. Tuan, Resorbable calcium sulfates with tunable degradation rate. J. Asian Ceram. Soc. 1(1), 102–107 (2013). https://doi.org/10.1016/j.jascer.2013.03.007
E. Shi, A. Wang, H. Li, R. Ogliore, Z. Ling, Gamma-CaSO\(_{4}\) with abnormally high stability from a hyperarid region on Earth and from Mars. J. Geophys. Res.: Planets 127, (2022). https://doi.org/10.1029/2021JE007108
T.M. Stawski, A.E.S. Van Driessche, R. Besselink, E.H. Byrne, P. Raiteri, J.D. Gale, L.G. Benning, The Structure of CaSO\(_{4}\) Nanorods: The Precursor of Gypsum. J. Phys. Chem. C (2019). https://doi.org/10.1021/acs.jpcc.9b04268
M.V. Thomas, D.A. Puleo, Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. B Appl. Biomater. 88B(2), 597–610 (2009). https://doi.org/10.1002/jbm.b.31269
M. Malik, M.A. Iqbal, J.R. Choi, P.V. Pham, 2D Materials for Efficient Photodetection: Overview, Mechanisms, Performance and UV-IR Range Applications. Front. Chem. 10, 905404 (2022). https://doi.org/10.3389/fchem.2022.905404
H. Nagabhushana, G. Nagaraju, B.M. Nagabhushana, C. Shivakumara, R.P.S. Chakradhar, Hydrothermal synthesis and characterization of CaSO\(_{4}\) pseudomicrorods. Philos. Mag. Lett. 90(4), 289–298 (2010). https://doi.org/10.1080/09500831003636051
T. Schmid, R. Jungnickel, P. Dariz, Insights into the CaSO\({4}\)-H\({2}\)O System: A Raman-Spectroscopic Study. Minerals 10(2), 115 (2020). https://doi.org/10.3390/min10020115
Acknowledgements
We express our gratitude to Professor Paulo de Tarso F. from Fortaleza, Brazil, for his valuable contributions to the Raman measurements and insightful discussions. We would also like to acknowledge the support provided by the UNAL Colombia-EMBRAPA-Brazil internationalization project.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Contributions
Angela M. Raba played a key role in synthesizing the samples and contributing to some of the writing, José Barba was involved in creating graphics and making corrections, and Miryam R. Joya conducted the analysis and contributed to the writing. The authors have thoroughly reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raba-Páez, A.M., Barba-Ortega, J. & Joya, M.R. Synthesis and morphological characterization of CaSO\(_{4}\): low-temperature Raman spectroscopy analysis of vibrational modes. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00701-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00701-5