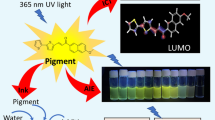
Abstract
A biocompatible fluorescent pigment, pyrene-pyridoxine azine (PyPy) derivative, has been designed that can display a vivid yellow hue under natural daylight and a cyano-green luminescence when exposed to UV light in solution. Interestingly, the same compound manifests a rich yellow color along with red fluorescence when integrated into solid-supported materials, like porous papers. Moreover, PyPy exhibits robust adherence to the paper surface, rapid drying, reasonable photostability, and considerable resistance to water, which can be considered advantageous for security-ink application. These observations strongly suggest that this probing molecule holds potential as a functional and environmentally friendly colorant for crafting water-based ink via flexography printing. Further, the notable high intensity of fluorescence, coupled with outstanding surface adhesion characteristics, facilitated the development of latent fingerprints (LFPs). These fingerprints, when illuminated, produce fluorescence images that offer distinct background contrast, thereby unveiling intricate level 1, level 2, and level 3 details on non-porous substrates that aid in individual identification. Moreover, the ink formulation of PyPy facilitated the identification of primary and secondary-level finger-mark details on both porous and non-porous surfaces. These results effectively showcase the successful utilization of the biocompatible PyPy fluorophore in fingerprint analysis, eliminating the necessity for additional adhesives and introducing a promising new tool for LFP detection.
Graphical Abstract









Similar content being viewed by others
Data availability
All data reported in this manuscript are available on request.
References
H. Chen, R.L. Ma, Y. Chen, L.J. Fan, Fluorescence development of latent fingerprint with conjugated polymer nanoparticles in aqueous colloidal solution. ACS Appl. Mater. Interfaces 9, 4908–4915 (2017)
S. Zhang, R. Liu, Q. Cui, Y. Yang, Q. Cao, W. Xu, L. Li, Covalent patterning and rapid visualization of latent fingerprints with photo-cross-linkable semiconductor polymer dots. ACS Appl. Mater. Interfaces 9, 44134–44145 (2017)
Y.L. Wang, C. Li, H.Q. Qu, C. Fan, P.J. Zhao, R. Tian, M.Q. Zhu, Real-time fluorescence in situ visualization of latent fingerprints exceeding level 3 details based on aggregation-induced emission. J. Am. Chem. Soc. 142, 7497–7505 (2020)
H. Zhang, J. You, C. Nie, J. Wang, X. Dong, R. Guan, D. Cao, Q. Chen, Non-conjugated organosilicone fluorescent nanoparticles for latent fingerprint detection. JOL 215, 116582 (2019)
C.F. Wang, R. Cheng, W.Q. Ji, K. Ma, L. Ling, S. Chen, Recognition of latent fingerprints, and ink-free printing derived from interfacial segregation of carbon dots. ACS Appl. Mater. Interfaces 10, 39205–39213 (2018)
E. Prabakaran, K. Pillay, Nanomaterials for latent fingerprint detection: a review. J. Mater. Res. Technol. 12, 1856–1885 (2021)
H. Chen, R.L. Ma, Y. Chen, L.J. Fan, fluorescence development of latent fingerprint with conjugated polymer nanoparticles in aqueous colloidal solution. ACS Appl. Mater. Interfaces 9, 4908–4915 (2017)
W. Chen, Y. Song, W. Zhang, R. Deng, Y. Zhuang, R.J. Xie, Time-gated imaging of latent fingerprints with level 3 details achieved by persistent luminescent fluoride nanoparticles. ACS Appl. Mater. Interfaces 14, 28230–28238 (2022)
J. Lian, F. Meng, W. Wang, Z. Zhang, Recent trends in fluorescent organic materials for latent fingerprint imaging. Front. Chem. 8, 594864 (2020)
H.Y. Wang, C.Y. Tan, K.K. Yu, K. Li, Y.H. Liu, X.Q. Yu, Multifunctional lipophilic purines: a coping strategy for anti-counterfeiting, lipid droplet imaging and latent fingerprint development. Mater. Chem. Front. 5, 6603–6610 (2021)
G.S. Sodhi, J. Kaur, Powder method for detecting latent fingerprints: a review. Forensic Sci. Int. 120, 172–176 (2001)
T. Xiao, L. Zhang, D. Chen, Q. Zhang, Q. Wang, Z.Y. Li, X.Q. Sun, A pillar[5]arene-based artificial light-harvesting system with red emission for high-resolution imaging of latent fingerprints. Org. Chem. Front. 10, 3245–3251 (2023)
M. Wang, D. Shen, Z. Zhu, J. Ju, J. Wu, Y. Zhu, M. Li, C. Yuan, C. Mao, Dual-mode fluorescent development of latent fingerprints using NaYbF4:Tm upconversion nanomaterials. Mater. Today Adv. 8, 100113 (2020)
M.K. Ravindra, G.P. Darshan, D.R. Lavanya, K.M. Mahadevan, H.B. Premkumar, S.C. Sharma, H. Adarsha, H. Nagabhushana, Aggregation induced emission based active conjugated imidazole luminogens for visualization of latent fingerprints and multiple anticounterfeiting applications. Sci. Rep. 11, 16748 (2021)
K.K. Sharma, G.H. Kannikanti, T.R. Baggi, J.R. Vaidya, A pyrene formulation for fluorometric visualization of latent fingermarks. Methods Appl. Fluoresc. 6, 035004 (2018)
H.S. Jung, K.J. Cho, S.J. Ryu, Y. Takagi, P.A. Roche, K.C. Neuman, Covalent patterning and rapid visualization of latent fingerprints with photo-cross-linkable semiconductor polymer dots. ACS Appl. Mater. Interfaces 12, 6641–6650 (2020)
I. Chang, A.C. Stone, O.C. Hanney, W.J. Gee, Volatilised pyrene: a phase 1 study demonstrating a new method of visualising fingermarks with comparisons to iodine fuming. Forensic Sci. Int. 305, 109996 (2019)
G.E. Florence, K.A. Bruce, H.J. Shepherd, W.J. Gee, Metastable 9-fluorenone: blueshifted fluorescence, single-crystal-to-single-crystal reactivity, and evaluation as a multimodal fingermark visualization treatment. Chem. A Eur. J. 25, 9597–9601 (2019)
K. Ayyavoo, P. Velusamy, Pyrene based materials as fluorescent probes in chemical and biological fields. New J. Chem. 45, 10997–11017 (2021)
M. Shellaiah, K.W. Sun, Pyrene-based AIE active materials for bioimaging and theranostics applications. Biosensors. 12, 550 (2022)
T.M. Figueira-Duarte, K. Mullen, Pyrene-based materials for organic electronics. Chem. Rev. 111, 7260–7314 (2011)
S. Paul, A. Ray Choudhury, N. Dey, Dual-Mode Multiple Ion Sensing via Analyte-specific modulation of Keto-Enol tautomerization of an ESIPT active pyrene derivative: experimental findings and computational rationalization. ACS omega 8, 6349–6360 (2023)
N. Dey, S. Bhattacharya, Trace level Al3+ detection in aqueous media utilizing luminescent ensembles comprising pyrene laced dynamic surfactant assembly. Dalton Trans. 47, 2352–2359 (2018)
S.L. Pilicer, P.R. Bakhshi, K.W. Bentley, C. Wolf, Biomimetic chirality sensing with pyridoxal-5′-phosphate. J. Am. Chem. Soc. 139, 1758–1761 (2017)
M. Chan-Huot, A. Dos, R. Zander, S. Sharif, P.M. Tolstoy, S. Compton, H.H. Limbach, NMR studies of protonation and hydrogen bond states of internal aldimines of pyridoxal 5′-phosphate acid–base in alanine racemase, aspartate aminotransferase, and poly-l-lysine. J. Am. Chem. Soc. 135, 18160–18175 (2013)
V. Bhardwaj, S.K. AK, S.K. Sahoo, Pyridoxal derived red-emitting aggregates for the ratiometric sensing of pH and copper (II). Opt. Mater. 136, 113414 (2023)
J. Mandal, N.C. Jana, S.G. Chowdhury, P. Karmakar, A. Saha, Two pyridoxal derived Schiff base chemosensors design for fluorescence sensing of Zn2+ ion in aqueous medium. Inorg. Chem. Commun. 156, 111217 (2023)
K. He, N. Chen, C. Wang, L. Wei, J. Chen, Method for determining crystal grain size by X-ray diffraction. Cryst. Res. Technol. 53, 1700157 (2018)
N. Dey, Coordination-driven reversible supramolecular assembly formation at biological pH: trace-level detection of Hg2+ and I− ions in real life samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 267, 120447 (2022)
R.S. Fernandes, N. Dey, Synthetic supramolecular host for D-(−)-ribose: ratiometric fluorescence response via multivalent lectin-carbohydrate interactions. ChemBioChem 23, e202200044 (2022)
V. Bhardwaj, A. Thangaraj, S. Varddhan, S.K.A. Kumar, G. Crisponi, S.K. Sahoo, An aggregation-induced emission active vitamin B6 cofactor derivative: application in pH sensing and detection of latent fingerprints. Photochem. Photobiol. Sci. 19, 1402–1409 (2020)
V. Kumar, P. Kumar, P. Kaur, K. Singh, A bis-pyrene chalcone based fluorescent material for ratiometric sensing of hydrazine: an acid/base molecular switch and solid-state emitter. Anal. Chim. Acta 1178, 338807 (2021)
R.S. Bhagya, D. Sunil, K. Muthamma, P. Shetty, D.S. Kulkarni, Water-based invisible green flexographic ink for anti-counterfeit applications. Prog. Org. Coat. 173, 107212 (2022)
H.L. Barros, M.A. Esteves, M.J. Brites, Synthesis, photophysical and electrochemical properties of π-conjugated pyrene based down-shifting molecules with fluorinated aryl groups. Dyes and Pigments 213, 111103 (2023)
Y. Ge, Y. Wen, H. Liu, T. Lu, Y. Yu, X.B. Li, S.W. Li, B. Yang, A key stacking factor for the effective formation of pyrene excimer in crystals: degree of π–π overlap. J. Mater. Chem. C 8, 11830–11838 (2020)
N. Dey, D. Biswakarma, A. Gulyani, S. Bhattacharya, Microenvironment sensitive charge-transfer dye for tandem sensing of multiple analytes at mesoscopic interfaces. ACS Sustain. Chem. Eng. 6, 12807–12816 (2018)
R.S. Fernandes, N. Dey, A combinatorial effect of TICT and AIE on bisulfate detection using a pyrenylated charge-transfer luminogen. Mater. Res. Bull. 163, 112192 (2023)
D. Devadiga, T.N. Ahipa, Protonation induced redshift in the fluorescence of a pyridine derivative as a potential anti-counterfeiting agent. Soft Matter 18, 8008–8016 (2022)
Z.D. Yu, X.X. Dong, J.Y. Cao, W.X. Zhao, G.H. Bi, C.Z. Wang, T. Zhang, S. Rahman, P.E. Georghiou, J.B. Lin, T. Yamato, Substituent effects on the intermolecular interactions and emission behaviors in pyrene-based mechanochromic luminogens. J. Mater. Chem. C 10, 9310–9318 (2022)
F. Liu, C. Tang, Q.Q. Chen, S.Z. Li, H.B. Wu, L.H. Xie, W. Huang, Pyrene functioned diarylfluorenes as efficient solution processable light emitting molecular glass. Organic Electronics 10, 256–265 (2009)
N. Dey, S. Bhattacharya, Mimicking multivalent protein–carbohydrate interactions for monitoring the glucosamine level in biological fluids and pharmaceutical tablets. Chem. Commun. 53, 5392–5395 (2017)
N. Dey, N. Kumari, D. Biswakarma, S. Jha, S. Bhattacharya, Colorimetric indicators for specific recognition of Cu2+ and Hg2+ in physiological media: effect of variations of signaling unit on optical response. Inorg. Chim. Acta 487, 50–57 (2019)
J. Li, Z. Jiao, P. Zhang, X. Wan, C. Song, Z. Guo, X. Huangi, B.Z. Tang, Development of AIEgen–montmorillonite nanocomposite powders for computer-assisted visualization of latent fingermarks. Mater. Chem. Front. 4, 2131–2136 (2020)
M. Rentzhog, Water-based flexographic printing on polymer-coated board; Chemical Science Engineering (Stockholm, KTH, 2006)
K. Muthamma, D. Sunil, P. Shetty, S.D. Kulkarni, P.J. Anand, D. Kekuda, Eco-friendly flexographic ink from fluorene-based Schiff base pigment for anti-counterfeiting and printed electronics applications. Prog. Org. Coat. 161, 106463 (2021)
D. Maltoni, D. Maio, A. K. Jain, J. Feng J (2022) Handbook of fingerprint recognition. Springer International Publishing, Cham.
J. Wang, Y. Gao, J. Zhang, H. Tian, Invisible photochromism, and optical anti-counterfeiting based on D–A type inverse diarylethene. J. Mater. Chem. C. 5, 4571–4577 (2017)
A.A. Ahmad, B. Workie, A.A. Mohamed, Diazonium gold salts as novel surface modifiers: what have we learned so far? Surfaces 3, 182–196 (2020)
S. Chen, K. Jia, Y. Fang, C. Liu, C. Yuan, J. Liu, K.P. Wang, Z.Q. Hu, Highly emissive dimethylamino naphthalenyl phenylethene derivatives for visualization of latent fingerprints and imaging of lysosomes. Dyes and Pigments 205, 110534 (2022)
K. Abhishek, A. Yogi, A minutiae count based method for fake fingerprint detection. Procedia Comput Sci 58, 447–452 (2015)
L.L. Da Luz, R. Milani, J.F. Felix, Inkjet printing of lanthanide–organic frameworks for anti-counterfeiting applications. ACS Appl. Mater. Interfaces 7, 27115–27123 (2015)
V. Vaibhav, U. Vijayalakshmi, S.M. Roopan, Agricultural waste as a source for the production of silica nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 139, 515–520 (2015)
K.A. Karthick, K. Kaleeswari, C. Uma Maheswari, G. Sivaraman, B. Shankar, A. Tamilselvi, Novel pyridoxal based molecular sensor for selective turn–on fluorescent switching functionality towards Zn(II) in live cells. J. Photochem. Photobiol. A 428, 113861 (2022)
O. Kocyigit, E. Guler, Synthesis of 1,3,5-tris(4-(4-nitrophenyliminomethyl)phenoxymethyl)benzene as a new Schiff base and its complexation properties with the (salen and salophen)-bridged Fe/Cr(III). J. Incl. Phenom. Macrocycl. Chem. 67, 287–293 (2010)
M. F. J. Bohan, P. Townsend, S. M. Hamblyn, T. C. Claypole, and D. T. Gethin, Evaluation of pressures in flexographic printing.
Author information
Authors and Affiliations
Contributions
The author (RSF) confirms the responsibility for the following: synthesis of compound and characterization, biocompatibility assay.
The authors (RKJ and KM) confirm the responsibility for the following: investigation, data collection, and validation of security ink formulation and LFP development studies.
The author (DS) confirms the responsibility for supervision, and writing—review and editing of security ink formulation and LFP development studies.
The author (ND) confirms the responsibility for the following: conceptualization, formal analysis, project administration, resources, software, supervision, visualization, writing—original draft, writing—review and editing of synthesis of compound, characterization, and biocompatibility assay.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
ESM 1
(DOCX 448 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sunil, D., Jain, R.K., Muthamma, K. et al. Pyrene-pyridoxine azine as a functional fluorophore: developing LFPs and formulating security ink. emergent mater. 7, 299–310 (2024). https://doi.org/10.1007/s42247-023-00594-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00594-w