Abstract
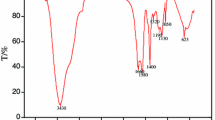
This article reports on poly (acrylamide-co-malonic acid) and poly(AM-co-MAc) copolymers that inhibit the iron sulfide scale. The iron sulfide scale is considered as one of the most challenging oilfield scales to dissolve or inhibit once formed due to the fast deposition kinetics and low solubility criteria. We present the synthesis, characterization, and testing of poly(AM-co-MAc) that inhibits iron sulfide formation by hindering nucleation growth and preventing particles from settling. A strictly anoxic apparatus was designed and used to evaluate the performance of the synthesized inhibitor. The influence of temperature, pH, and brine composition was also quantified. The results indicated that poly(AM-co-MAc) inhibits iron sulfide formation and nucleation growth under various conditions. The inhibitor effectively maintained iron sulfide particle separation at specific dosages, lowering its ability to cluster. Maximum efficiency of 92% was recorded at 60 °C and 1% volume inhibitor concentration. DFT calculations confirmed the inhibitive properties of poly(AM-co-MAc) via binding energy calculations. Compared to recently reported inhibitors, poly(AM-co-MAc) proves to be safer concerning toxicity properties. This study provides theoretical and technical insights for a novel chemistry solution for the metal-sulfide scale.












Similar content being viewed by others
References
A. O. James and N. B. Iroha, New green inhibitor of Olax subscorpioidea root for J55 carbon steel corrosion in 15% HCl: theoretical, electrochemical, and surface morphological investigation. Emergent Mater. 2021 54, 5(4) 1119–1131 (2021). https://doi.org/10.1007/S42247-021-00161-1
M. S. Kamal, I. Hussein, M. Mahmoud, A. S. Sultan, and M. A. S. Saad, Journal of petroleum science and engineering oil fi eld scale formation and chemical removal : a review. 171, 127–139 (2018). https://doi.org/10.1016/j.petrol.2018.07.037
P. Zhang, D. Shen, G. Ruan, A.T. Kan, M.B. Tomson, Phosphino-polycarboxylic acid modified inhibitor nanomaterial for oilfield scale control: synthesis, characterization and migration. J. Ind. Eng. Chem. 45, 366–374 (2017). https://doi.org/10.1016/J.JIEC.2016.10.004
S. Kumar, T.K. Naiya, T. Kumar, Developments in oilfield scale handling towards green technology-A review. J. Pet. Sci. Eng. 169, 428–444 (2018). https://doi.org/10.1016/J.PETROL.2018.05.068
A. N. El-hoshoudy, A. Ghanem, and S. M. Desouky, “Imidazolium-based ionic liquids for asphaltene dispersion; experimental and computational studies,” J. Mol. Liq., vol. 324, p. 114698, Feb. 2021, doi: https://doi.org/10.1016/J.MOLLIQ.2020.114698.
T. Chen, H. Montgomerie, P. Chen, T. Hagen, and S. Kegg, Development of environmental friendly iron sulfide inhibitors for field application. Proc. - SPE Int. Symp. Oilf. Chem. 1, 259–268 (2009). https://doi.org/10.2118/121456-MS
K.D. Demadis, Z. Anagnostou, H. Zhao, Novel calcium carboxyphosphonate/polycarboxylate inorganic-organic hybrid materials from demineralization of calcitic biomineral surfaces. ACS Appl. Mater. Interfaces 1(1), 35–38 (2009). https://doi.org/10.1021/AM800030H/SUPPL_FILE/AM800030H_SI_002.PDF
K. Chauhan, P. Sharma, and G. S. Chauhan, Removal/dissolution of mineral scale deposits. Miner. Scales Depos. Sci. Technol. Approaches 701–720 (2015). https://doi.org/10.1016/B978-0-444-63228-9.00029-2
B.G. Al-Harbi, A.J. Graham, K.S. Sorbie, Iron sulfide inhibition and interaction with zinc and lead sulfides. SPE Prod. Oper. 34(03), 551–563 (2019). https://doi.org/10.2118/190743-PA
C. Okocha and K. Sorbie, “Scale prediction for iron, zinc and lead sulphides and its relation to scale test design.” OnePetro, Mar. 09, 2014.
J. J. Wylde, C. Okocha, M. Bluth, A. Savin, and B. Adamson, Iron sulfide inhibition: field application of an innovative polymeric chemical. Proc. - SPE Int. Symp. Oilf. Chem. 1, 302–320 (2015). https://doi.org/10.2118/173730-MS
M. Goyal, S. Kumar, I. Bahadur, C. Verma, E.E. Ebenso, Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 256, 565–573 (2018). https://doi.org/10.1016/J.MOLLIQ.2018.02.045
M. Askari, M. Aliofkhazraei, R. Jafari, P. Hamghalam, and A. Hajizadeh, Downhole corrosion inhibitors for oil and gas production – a review. Appl. Surf. Sci. Adv., 6, 100128 (2021). https://doi.org/10.1016/J.APSADV.2021.100128
A.M. Al-Sabagh, N.M. El Basiony, S.A. Sadeek, M.A. Migahed, Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: experimental, and theoretical investigations. Desalination 437, 45–58 (2018). https://doi.org/10.1016/J.DESAL.2018.01.036
Y. Ji et al., Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate. Desalination 422, 165–173 (2017). https://doi.org/10.1016/J.DESAL.2017.08.027
A. Hamza, I. A. Hussein, R. Jalab, M. Saad, and M. Mahmoud, “Review of iron sulfide scale removal and inhibition in oil and gas wells: current status and perspectives,” https://doi.org/10.1021/acs.energyfuels.1c02177.
M.S. Kamal, I. Hussein, M. Mahmoud, A.S. Sultan, M.A.S. Saad, Oilfield scale formation and chemical removal: a review. J. Pet. Sci. Eng. 171, 127–139 (2018). https://doi.org/10.1016/J.PETROL.2018.07.037
M.F. Mady, M.A. Kelland, Review of nanotechnology impacts on oilfield scale management. ACS Appl. Nano Mater. 3(8), 7343–7364 (2020). https://doi.org/10.1021/ACSANM.0C01391
A.A. Olajire, A review of oilfield scale management technology for oil and gas production. J. Pet. Sci. Eng. 135, 723–737 (2015). https://doi.org/10.1016/J.PETROL.2015.09.011
D. Hasson, H. Shemer, A. Sher, State of the art of friendly ‘green’ scale control inhibitors: a review article. Ind. Eng. Chem. Res. 50(12), 7601–7607 (2011). https://doi.org/10.1021/IE200370V
M.F. Mady, M.A. Kelland, Overview of the synthesis of salts of organophosphonic acids and their application to the management of oilfield scale. Energy Fuels 31(5), 4603–4615 (2017). https://doi.org/10.1021/ACS.ENERGYFUELS.7B00708
M. Mpelwa and S.-F. Tang, State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: a review. Pet. Sci. 2019 164, 16(4), 830–849 (2019). https://doi.org/10.1007/S12182-019-0299-5
N. Bhandari et al., Identification of new chemical formulation for control of metal sulfides FeS, ZnS and PbS scale deposition. Proc. - SPE Int. Symp. Oilf. Chem., 2017, 294–305 (2017). https://doi.org/10.2118/184526-MS
W. Li et al., Development of novel iron sulfide scale control chemicals. Soc. Pet. Eng. - SPE Int. Oilf. Scale Conf. Exhib. 2018, (2018). https://doi.org/10.2118/190755-ms
S. Ko, X. Wang, A. T. Kan, and M. B. Tomson, “Identification of novel chemicals for iron sulfide scale control and understanding of scale controlling mechanism,” in Proceedings - SPE International Symposium on Oilfield Chemistry, 2019, (2019)., https://doi.org/10.2118/193550-ms
B. Alharbi, N. Aljeaban, A. Graham, and K. S. Sorbie, Iron sulfide and zinc sulfide inhibition and scale inhibitor consumption. Soc. Pet. Eng. - Abu Dhabi Int. Pet. Exhib. Conf. 2019, ADIP 2019, (2019). https://doi.org/10.2118/197688-ms
N. Bhandari, M. Bhandari, I. Littlehales, and J. Fidoe, Development of a novel iron sulfide scale inhibitor for onshore US application. Proc. - SPE Int. Symp. Oilf. Chem. 2019, (2019). https://doi.org/10.2118/193599-ms
Y. Xu, L.L. Zhao, L.N. Wang, S.Y. Xu, Y.C. Cui, Synthesis of polyaspartic acid–melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide. Desalination 286, 285–289 (2012). https://doi.org/10.1016/J.DESAL.2011.11.036
M.K. Jensen, M.A. Kelland, A new class of hyperbranched polymeric scale inhibitors. J. Pet. Sci. Eng. 94–95, 66–72 (2012). https://doi.org/10.1016/J.PETROL.2012.06.025
X. Guo et al., Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan. J. Ind. Eng. Chem. 18(6), 2177–2183 (2012). https://doi.org/10.1016/J.JIEC.2012.06.015
X. Liu, K. Liu, S. Gou, L. Liang, C. Luo, Q. Guo, Water-soluble acrylamide sulfonate copolymer for inhibiting shale hydration. Ind. Eng. Chem. Res. 53(8), 2903–2910 (2014). https://doi.org/10.1021/IE403956D/ASSET/IMAGES/LARGE/IE-2013-03956D_0006.JPEG
S. Kamali, R. Arefinia, Effect of PAAT as an environmentally friendly terpolymer on the scale inhibition of CaCO3 in artificial seawater: chemical and electrochemical study. Ind. Eng. Chem. Res. 59(2), 627–635 (2020). https://doi.org/10.1021/ACS.IECR.9B05943/ASSET/IMAGES/LARGE/IE9B05943_0009.JPEG
C. Cui, S. Zhang, Preparation, characterization and performance evaluation of a novel scale inhibiting and dispersing copolymer containing natural tannin. J. Polym. Environ. 28(7), 1869–1879 (2020). https://doi.org/10.1007/S10924-020-01730-X/FIGURES/9
D.E. Abd-El-Khalek, H.H.A.M. Hassan, S.R. Ramadan, Water-soluble sulfonated polyaniline as multifunctional scaling inhibitor for crystallization control in industrial applications. Chem. Eng. Res. Des. 169, 135–141 (2021). https://doi.org/10.1016/J.CHERD.2021.03.004
W.G.P. Kumari et al., Hydraulic fracturing under high temperature and pressure conditions with micro CT applications: geothermal energy from hot dry rocks. Fuel 230, 138–154 (2018). https://doi.org/10.1016/J.FUEL.2018.05.040
H. Wang, J. Hu, Z. Yang, Z. Yin, Q. Xiong, X. Zhong, The study of a highly efficient and environment-friendly scale inhibitor for calcium carbonate scale in oil fields. Petroleum 7(3), 325–334 (2021). https://doi.org/10.1016/J.PETLM.2021.01.005
L. Yang et al., Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 416, 166–174 (2017). https://doi.org/10.1016/J.DESAL.2017.05.010
L.-C. Wang et al., Evaluation of maleic acid-based copolymers containing polyoxyethylene ether as inhibitors for CaCO3 scale. J. Appl. Polym. Sci. 136(19), 47470 (2019). https://doi.org/10.1002/APP.47470
K. ‐J Yao and F. ‐H Liu, Synthesis and rheological behavior in aqueous solutions of poly(acrylamide-co-maleic acid). J. Appl. Polym. Sci. 56(1), 9–15 (1995). https://doi.org/10.1002/APP.1995.070560102
Y. M. Mohan, K. Sudhakar, P. S. K. Murthy, and K. M. Raju, Swelling properties of chemically crosslinked poly(acrylamide-co-maleic acid) hydrogels, 55(7), 513–536 (2007). https://doi.org/10.1080/00914030500208246
D. Saraydin, E. Karada ˘ G, N. Sahiner, and O. G. ¨ Uven, “Incorporation of malonic acid into acrylamide hydrogel by radiation technique and its effect on swelling behavior.”
M. J. Frisch et al., “Gaussian 09, revision D.01,” Gaussian, Inc. Wallingford CT. Gaussian, Inc. TS - CrossRef Metadata Search, Wallingford CT, 2013, https://doi.org/10.1063/PT.4.2023 M4 - Citavi
K.O. Sulaiman, A.T. Onawole, D.T. Shuaib, T.A. Saleh, Quantum chemical approach for chemiluminescence characteristics of di-substituted luminol derivatives in polar solvents. J. Mol. Liq. 279, 146–153 (2019). https://doi.org/10.1016/j.molliq.2019.01.110
I.B. Obot, K. Haruna, T.A. Saleh, Atomistic simulation : a unique and powerful computational tool for corrosion inhibition research. Arab. J. Sci. Eng. (2018). https://doi.org/10.1007/s13369-018-3605-4
I.B.B. Obot, D.D.D. Macdonald, Z.M.M. Gasem, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: Part 1: an overview. Corros. Sci. 99, 1–30 (2015). https://doi.org/10.1016/j.corsci.2015.01.037
D. K. Verma, “Density functional theory (DFT) as a powerful tool for designing corrosion inhibitors in aqueous phase,” in Advanced Engineering Testing, 2018.
J. Laun, T. Bredow, BSSE-corrected consistent Gaussian basis sets of triple-zeta valence with polarization quality of the sixth period for solid-state calculations. J. Comput. Chem. 42(15), 1064–1072 (2021)
A.V. Marenich, C.J. Cramer, D.G. Truhlar, Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113(18), 6378–6396 (2009). https://doi.org/10.1021/jp810292n
T. K. R. Dennington J. Millam, R. Dennington, T. Keith, and J. Millam, “Gaussview,” Semichem Inc, Shawnee Mission KS, 131 TS-, (37), (2009). https://doi.org/10.1021/ja906885v M4 - Citavi
S.K. Bajpai, S. Johnson, Poly(acrylamide-co-maleic acid) hydrogels for removal of Cr(VI) from aqueous solutions, part 1: synthesis and swelling characterization. J. Appl. Polym. Sci. 100(4), 2759–2769 (2006). https://doi.org/10.1002/APP.23420
L. Aref, A.H. Navarchian, D. Dadkhah, Adsorption of crystal violet dye from aqueous solution by poly(acrylamide-co-maleic acid)/montmorillonite nanocomposite. J. Polym. Environ. 25(3), 628–639 (2017). https://doi.org/10.1007/S10924-016-0842-Z/FIGURES/8
E. K. Karadagˇ*, D. Saraydın, and O. Güven, “Radiation induced superabsorbent hydrogels. acrylamide/itaconic acid copolymers,” https://doi.org/10.1002/1439-2054
Y. He et al., “Thermoresponsive behaviors of novel polyoxyethylene-functionalized acrylamide copolymers: water solubility, rheological properties and surface activity,” J. Mol. Liq., vol. 319, p. 114337, Dec. 2020, doi: https://doi.org/10.1016/J.MOLLIQ.2020.114337.
W.M. Leung, D.E. Axelson, J.D. Van Dyke, Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J. Polym. Sci. Part A Polym. Chem. 25(7), 1825–1846 (1987). https://doi.org/10.1002/POLA.1987.080250711
T. Begam, R.S. Tomar, A.K. Nagpal, R. Singhal, Synthesis of poly(acrylamide-co-methyl methacrylate-co-vinyl amine-co-acrylic acid) hydrogels by Hoffman degradation and their interactions with acetaminophen. J. Appl. Polym. Sci. 94(1), 40–52 (2004). https://doi.org/10.1002/APP.20706
T. Hoare, R. Pelton, Functional group distributions in carboxylic acid containing poly(n-isopropylacrylamide) microgels. Langmuir 20(6), 2123–2133 (2004). https://doi.org/10.1021/LA0351562/ASSET/IMAGES/MEDIUM/LA0351562E00006.GIF
Y. Liao, H. Zheng, L. Qian, Y. Sun, L. Dai, and W. Xue, UV-initiated polymerization of hydrophobically associating cationic polyacrylamide modified by a surface-active monomer: a comparative study of synthesis, characterization, and sludge dewatering performance. (2014). https://doi.org/10.1021/ie5016987
S.K. Rath, R.P. Singh, On the characterization of grafted and ungrafted starch, amylose, and amylopectin. J Appl Polym Sci 70, 1795–1810 (1998). https://doi.org/10.1002/(SICI)1097-4628(19981128)70:9
M. Pandey, N. Mohamad, M. Cairul, and I. M. Amin, “Bacterial cellulose/acrylamide pH-sensitive smart hydrogel: development, characterization, and toxicity studies in ICR mice model,” 2014, doi: https://doi.org/10.1021/mp500337r.
R. Bisatto, V. M. Picoli, and C. L. Petzhold, “Evaluation of different polymeric scale inhibitors for oilfield application,” J. Pet. Sci. Eng., vol. 213, p. 110331, Jun. 2022, doi: https://doi.org/10.1016/J.PETROL.2022.110331.
A.I.A. Mohamed, I.A. Hussein, A.S. Sultan, G.A. Al-Muntasheri, Gelation of emulsified polyacrylamide/polyethylenimine under high-temperature, high-salinity conditions: rheological investigation. Ind. Eng. Chem. Res. 57(36), 12278–12287 (2018). https://doi.org/10.1021/ACS.IECR.8B02571
A. Mansri, A. Bendraoua, A. Benmoussa, K. Benhabib, New polyacrylamide [PAM] material formulations for the coagulation/flocculation/decantation process. J. Polym. Environ. 23(4), 580–587 (2015). https://doi.org/10.1007/S10924-015-0734-7/FIGURES/8
D.R. Biswal, R.P. Singh, Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 57(4), 379–387 (2004). https://doi.org/10.1016/J.CARBPOL.2004.04.020
S. Baraka-Lokmane, K.S. Sorbie, Effect of pH and scale inhibitor concentration on phosphonate–carbonate interaction. J. Pet. Sci. Eng. 70(1–2), 10–27 (2010). https://doi.org/10.1016/J.PETROL.2009.05.002
C. Fan et al., Scale prediction and inhibition for oil and gas production at high temperature/high pressure. SPE J. 17(02), 379–392 (2012). https://doi.org/10.2118/130690-PA
R. Jalab, M.A. Saad, I.A. Hussein, A.T. Onawole, Calcite scale inhibition using environmental-friendly amino acid inhibitors: DFT investigation. ACS Omega 6(47), 32120–32132 (2021). https://doi.org/10.1021/acsomega.1c04888
X. Chen, Y. Chen, J. Cui, Y. Li, Y. Liang, and G. Cao, Molecular dynamics simulation and DFT calculation of ‘green’ scale and corrosion inhibitor. Comput. Mater. Sci. 188(2020), 110229 (2021). https://doi.org/10.1016/j.commatsci.2020.110229
G. Xiong et al., ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 49(W1), W5–W14 (2021). https://doi.org/10.1093/nar/gkab255
J.S. Delaney, ESOL: estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 44(3), 1000–1005 (2004). https://doi.org/10.1021/ci034243x
Acknowledgements
The authors would like to acknowledge the City of Grand Forks for funding this study through Project Number: UND0023658 and Qatar University National Capacity Building Program (NCBP), grant #QUCP-CENG-2021-03. The Research Computing group from Texas A&M University at Qatar provided the HPC resources and services used in this work. The Qatar Foundation funds research computing for education, science, and community development (http://www.qf.org.qa). The outcomes achieved are exclusively the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ismail, N., Alshami, A., Jalab, R. et al. Synthesis and performance evaluation of poly (acrylamide-co-malonic acid) as FeS scale inhibitor: experimental and theoretical investigations. emergent mater. 7, 495–508 (2024). https://doi.org/10.1007/s42247-023-00456-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00456-5