Abstract
An experimental detail on the morphology engineering and characterizations of the all-inorganic Sn-based perovskite (here CsSnI3) thin films and their application in photodetectors are presented. In particular, we demonstrated that the chlorobenzene anti-solvent treatment during thin-film spin coating could effectively optimize the morphology properties of the obtained CsSnI3 thin film. SEM and AFM measurements showed the uniform thin film with nanorod-like nanocrystalline morphology. In addition, EDS and XPS measurements confirmed the low level of oxidation of the thin film, indicating good ambient stability. A planar photodetector was also made with the prepared thin film, and electrical characteristics were taken. The dark current and photocurrent were found in the range of 10−9 A and 10−7 A, respectively, with an on/off ratio of 102. The photoresponsivity was 10−5 AW−1. A further experiment was conducted to make composite thin films between CsSnI3 and CNTs for additional morphological engineering. The SEM measurement and Raman mapping manifested the nanonet-like morphology of the composite thin film. The quenching of the photoluminescence curve indicated the efficient photo-generated carrier extraction from the CsSnI3 matrix to CNTs. The absorption spectra also showed enhanced absorption ability of the prepared composite thin film. A hybrid photodetector made from the composite thin film showed dark current and photocurrent in the range of 10−6 A and 10−4 A, respectively, with an on/off ratio of 102. The photoresponsivity was 10−2 AW−1. Due to the combination of the CNTs with CsSnI3, the photoresponsivity increased 1000 times. At the same time, the hysteresis of the hybrid photodetector also reduced significantly compared to the pristine CsSnI3-based photodetector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
All-inorganic perovskites (AIPs) are becoming a class of materials of interest due to their improved stability property as compared to the organic–inorganic hybrid perovskites (OIHPs) in ambient conditions. For example, they have been proved that the replacements of organic cations like methylammonium (MA, CH3NH3) with inorganic cation Cesium (Cs) can enhance the thermal stability of perovskites [1]. Moreover, considering the future environmental hazards of toxic lead (Pb)-based halide perovskites for optoelectronic devices, it is essential to find alternative lower toxic metal-based perovskites. In this context, recent research works have found that Sn can be the most suitable one among various possible alternatives, such as bismuth (Bi) [2, 3], copper (Cu) [4], germanium (Ge) [5], antimony (Sb) [6, 7], and so on [8], for the non-Pb-based halide perovskites. The convincing reason for choosing Sn is that they have a very similar atomic radius (Sn2+:1.35 Å; and Pb2+:1.49 Å) as that of Pb [9] and also a similar electronic configuration (ns2np2) [10] between Sn and Pb. In this sense, it is expected that the Sn-based perovskites would have similar characteristics as the Pb-based perovskites. Furthermore, Sn-based perovskites have some other advantages over Pb-based perovskites for developing optoelectronic devices, especially photodetectors. Sn-based perovskites have lower bandgaps as compared to Pb-based perovskites. For example, Sn-based hybrid perovskite FASnI3 has a low bandgap of 1.41 eV [11] (FA = formamidinium), and the AIP such as CsSnI3 has a much lower bandgap of 1.3 eV [12]. They also have a high optical absorption coefficient and low exciton binding energy, favorable for optoelectronic devices [13,14,15,16]. Moreover, Sn-based perovskites have also been demonstrated to have higher carrier motilities than Pb-based perovskites. Hall effect measurements regarding the black orthorhombic perovskite phase of CsSnI3 indicated that the high hole mobility of about 585 cm2V−1 s−1 can be obtained, which is one of the highest values among different p-type semiconductors [13]. Last but not least, Sn-based perovskites also have higher thermodynamic and chemical stability than the Pb-based perovskites [17].
Despite the promising advantages of the Sn-based perovskites for optoelectronics, there are still some challenges in implementing all-inorganic CsSnI3-based optoelectronic devices. The Goldschmidt tolerance factor t for perovskite materials with the formula of ABX3 can be expressed as t = (rA + rX)/(√2 × [rB + rX]) [18], where rA, rB, and rX are the ionic radii of the A, B, and X ions, respectively. According to the tolerance factor (t), the inclusion of Cs+ cation makes the lower limit (typically 0.81 < t < 1.11), which makes the perovskite CsSnI3 very unstable; that is, it can transform into non-optically active non-perovskites (orthorhombic yellow phase) from optically active perovskites (orthorhombic black phase) at room temperature [19,20,21]. Moreover, the Sn2+ state of CsSnI3 converts to Sn4+ state when exposed to the ambient atmosphere [22]. CsSnI3 also has a large number of Sn vacancies that act as the p-type dopants and the recombination centers. The p-type dopants create highly mobile holes, and CsSnI3 can thus transform metallic from semiconducting [13]. Due to the above disadvantages, the CsSnI3-based optoelectronic devices are also suffering from poor device performance, which would thus require further developments. Although there are a few works on CsSnI3-based photovoltaics [15, 23,24,25,26], photodetectors [27, 28], and other optoelectronic devices [29], great efforts are still needed to further address the above disadvantages, aiming for exploring high-performance CsSnI3-based optoelectronic devices with the improved stability.
To date, various strategies, like adding different additives, have been applied to improve the performance and stability of the Sn-based perovskite optoelectronic devices. To further upgrade the device performance, it is also obligatory to reduce the Sn vacancies and oxidation. For Sn-based perovskite optoelectronic devices, the high-quality film with fewer defects is important because that means the lower Sn vacancies and the good-quality film can prevent oxygen and moisture from penetrating its internal region, which can reduce the oxidation of Sn2+. Ascorbic acid is an antioxidant solution additive [27], which can not only prevent the oxidation of Sn2+ but also control the perovskites’ crystallization significantly, and thus improve the optoelectronic device performance. For example, Xu et al. found a prolonged photo-generated carrier lifetime (182.7 ns) of the ascorbic acid-treated MA0.5FA0.5Pb0.5Sn0.5I3 thin films [30]. In addition, adding Sn-based halides SnX2 (X = F, Cl, Br, I) can also reduce the Sn vacancies and oxidation [31]. In this regard, Heo et al. also proved that adding SnX2 (X = F, Cl, Br) could benefit passivating the surface effectively [16]. They achieved excellent stability property of about 100-h operation for the CsSnI3-based devices with the addition of SnBr2. Marshall et al. found that the excessive use of SnI2 during the synthesis process of CsSnI3 can improve the materials’ stability and the related photovoltaic device performance [32]. The same group further demonstrated that the addition of SnCl2 in the precursor solution could also enhance the photovoltaic device stability and performance in ambient conditions [15]. They also observed that SnCl2 could form as a thin film or layer of particles on the surface of the CsSnI3 polycrystalline thin film. The SnCl2 surface layer can work as a desiccant and sacrificial agent because SnCl2 film absorbs H2O to form a stable hydrate (SnCl2·2H2O), which is then oxidized to SnO2. Several groups also tried to add SnF2 with the Sn-based perovskite precursor, aiming to improve the sample quality and the related device performance. Kumar et al. [33] found that the addition of SnF2 in the precursor solution of CsSnI3 during the synthesis process could reduce the metallic property of the obtained CsSnI3. They observed the clear conductivity reduction behavior, which indicates the reduction of Sn vacancies in the obtained Sn-based perovskite samples. Koh et al. [11] observed that the added SnF2 could prevent the oxidation of Sn2+ and further recover the substrate coverage of hybrid perovskite FASnI3. Xiao et al. [34] explored the effects of the SnF2 additive on MASnIBr2 films with different concentrations of SnF2. They discovered that the precipitated SnF2 also has the function of producing more crystal growth cores, and ultimately the film can become more uniform with the enriched coverage. Liao et al. [35] noticed that the pinholes in the FASnI3 film disappeared, and the film became denser and more uniform with increasing the concentration of the adding SnF2. Zhao et al. [36] added 10 mol% SnF2 additives for growing Sn-based hybrid perovskite FA0.75MA0.25SnI3 films, and they achieved better optoelectronic performance and enhanced stability in the nitrogen environment. It should be noted that the Sn vacancies can create natural defects at grain boundaries and on surfaces of the Sn-based perovskite films. In this regard, an effective strategy to passivate these naturally formed defects should be explored to incorporate organic chain polymers into the polycrystalline thin film. Deng et al. utilized a Lewis-type organic insulating polymer polymethylmethacrylate (PMMA) as an effective additive in FASnI3 precursor to optimize the surface morphology and reduce the trap density [37]. Besides, antisolvent also plays the crucial role in improving the morphology of Sn-based perovskites. In this perspective, Liu et al. verified the effects of different antisolvents on Sn-based perovskite film formation. They prepared FA0.75MA0.25SnI3 through using diethyl ether, toluene, and chlobenzene as the antisolvents, and they observed that chlorobenzene was the best antisolvent to obtain a dense and uniform Sn-based perovskite film [38].
Recently, tin (Sn)-based all-inorganic perovskites (AIPs) have shown significant potential for various optoelectronic applications, especially the photodetectors, due to their suitable bandgap low exciton binding energy, and the predicted high carrier mobility, etc. [39]. Unfortunately, the device performance of the photodetectors based on these emerging AIPs is still limited by their strong hysteresis effect and low carrier mobility [40]. The bitter truth is that the anomalous current–voltage (I-V) hysteresis behaviors with the different voltage scanning directions are still not fully understood yet in halide perovskites. Recently, a couple of popular underlying factors were found in different pieces of literature, such as ferroelectricity due to changing polarization with external switching field [41, 42], ion migration with interfacial field change and ion accumulation at interfaces [43,44,45], charge tunneling due to local heavy doping originated from the electrostatic dipole at a rough interface [46], and charge scattering by trapping and de-trapping at grain boundaries and other defects like pinholes, which can also lead to the experimentally obtained low carrier mobility values [47,48,49,50]. Therefore, coupling halide perovskite films with other functional materials with high carrier mobilities should be one of the effective methods to enhance optoelectronic device performance by improving carrier transport properties and suppressing hysteresis. In this regard, incorporating Sn-based perovskites with carbon nanomaterials (CNMs) for preparing the hybrid composites can be an ideal approach. CNMs could serve as effective charge transport pathways by reducing the grain boundaries and pinholes in Sn-based perovskite films. Among various carbon nanomaterials, graphene and carbon nanotubes (CNTs) are more suitable due to their fantastic charge transport properties. The optoelectronic devices made by the hybrid films that combinate the halide perovskites with graphene have been demonstrated to show good transport properties; however, the dark currents were too high in these composite samples, due to the high conductivity of graphene, which would affect their photo-response performance [51,52,53,54]. Although it was also reported that adding another kind of common CNMs like fullerenes could effectively reduce the hysteresis effect in the halide perovskite optoelectronic devices [55, 56], such the approach has mainly been employed for the organic–inorganic hybrid perovskites (OIHPs) or AIP quantum dots with the organic ligand-wrapped, which can facilitate the marching interface between each other. In comparison, semiconducting CNTs were also successfully used to hybridize with other materials, like metal oxides, quantum dots, or organic semiconductors, for improving the related device performance, thanks to their outstanding electronic and mechanical characteristics [57]. Notably, the semiconducting CNTs also have promising optoelectronic characteristics, including the low direct bandgap and high carrier mobility, which are necessary and helpful for the high-performance photodetector applications based on halide perovskite materials. More importantly, the semiconducting CNTs have excellent carrier transport features, particularly the ultrahigh carrier mobility of up to 10,000 cm2V–1s–1[58]. In this regard, as for the relevant hybrid composites, the added semiconducting CNTs can offer highway-like transport paths, enabling smoother charge carrier transport with less trapping and scattering within the composite materials. The addition of semiconducting CNT also did not change the crystalline property and optoelectronic features of halide perovskites [59]. Previous publications have demonstrated that CNTs can be used as electron transport layer, hole transport layer, or electrodes in the halide perovskite solar cells and other optoelectronic devices, which played an essential role in improving the device performance [59]. The composite materials containing CNTs and halide perovskites and the related devices have been investigated in a couple of works [60, 61]; however, the main obstacle to preparing the perovskite/CNT composite solutions is still placed on the aggregation of CNTs in the perovskite precursor. This might be attributed to the strong intra-tube van der Waals interaction between the individual CNTs, which can turn them into bundles and thus hinder their even distribution in the solution [62].
Here, the precursor solution of CsSnI3 was prepared with ascorbic acid to reduce oxidation, while the extra SnF2 and Sn powder were used to minimize Sn vacancies, and PMMA was employed to reduce grain boundaries. At the same time, during the perovskite thin-film spin coating, different antisolvents like toluene, ethyl acetate, and chlorobenzene were used for getting good film crystallization. So far, all the reported studies on the all-inorganic CsSnI3-based photodetection devices were carried out under a nitrogen atmosphere in a glovebox. On the other hand, for promoting the practical applications and the commercial production of those devices, the fabrication process of these perovskites and the related devices in the ambient condition is highly required, which can reduce the cost. Here we developed the preparation and the optimization strategies for the high-quality CsSnI3 films in completely open-air conditions. We found some unique features, like nanorod-like thin-film morphology of the obtained films, as well as the improved ambient stability and more negligible hysteresis for the related devices, etc. We also developed a photodetector based on this new morphology AIP and measured their optoelectronic performance. The dark current was found to be around 10−9 A, and the obtained photocurrent is about 10−7 A. Then, the photo-responsivity of the fabricated photodetector was calculated, which reached 10−5 A W−1, although it was not too high. The on/off ratio between the currents in the dark and under light, respectively, of the photodetector was about 102. It is hoped that this work will provide insight into the CsSnI3-based photodetectors and will help with explaining some of the issues about these photodetectors for their future development.
In addition, the photodetector devices based on the composite films that incorporate all-inorganic CsSnI3 perovskite and (6,5) semiconducting CNTs are investigated and reported. The resultant perovskite/CNTs composite thin films were successfully synthesized through a facile solution-processed method. In particular, the CNTs were polymer (poly fluorene class) wrapped to remove the problem of aggregation. The CsSnI3/CNT composite thin films were prepared via the spin-coating method in the open-air condition. The SEM measurements and Raman mapping were carried out to investigate the thin-film morphology and compositional properties. A nanonet type of morphology was obtained. Photoluminescence (PL) spectra and absorption spectra were also done to study optical properties. The hybrid photodetectors based on the obtained composite thin film were fabricated, and their electrical and photodetection performance was evaluated. The optoelectronic performance was significantly improved compared to the pristine CsSnI3 thin film. The photodetector fabricated with the composite film offered a dark current in the 10−6 A range and a photocurrent in the 10−4 A range. The on/off ratio was 102. The responsivity was in the range of 10−2 AW−1. These research findings will provide insights into the CsSnI3/CNT composite thin films and their applications in photodetectors and will be helpful for the future development of composite material–based optoelectronic devices.
2 Materials for thin-film preparation
The required materials for the perovskite precursor include CsI (cesium iodide: anhydrous, powder, 99.99% trace metal basis), and SnI2 (tin (II) iodide: anhydrous, powder, 99.99% trace metal basis). The required additives that we used in our case are SnF2 (tin (II) fluoride: anhydrous, powder, 99.99% trace metal basis), Sn powder (150 µm, 99.50% trace metal basis), ascorbic acid (99.99%), and PMMA (poly(methyl methacrylate): average Mw ~ 120,000 by GPC). DMSO (dimethyl sulfoxide: anhydrous, 99.90%) was used as a solvent. CNT (95% carbon basis, (6,5) chirality, and 0.78 nm average diameter) and PFDD (poly(9,9-di-n-dodecylfluorene) were also used. The required antisolvent in our experiment is shown here: toluene (anhydrous, 99.90%), ethyl acetate (anhydrous, 99.8%), and chlorobenzene (HPLC, 99.90%), which were all purchased from Sigma-Aldrich P/L. Glass substrates were bought from Great Cell Solar Australia Pty Ltd.
3 Thin-film preparation and relevant photodetector fabrication
At first, two small glass vials (10 ml each) were taken. The small glass vials were washed in a bath sonicator. The glass vials were washed with acetone, ethanol, and propanol, for 5 min each. The water temperature was raised to about 30 °C at the time of sonication. Then the vials were kept for drying for about 30 min in a vacuum oven at the temperature of 100 °C. Then they were also dried using the flow of N2 gas. Then we prepared the all-inorganic CsSnI3 solution precursor with a concentration of 1.5 M. To prepare the high-quality precursor, the following raw materials with the stoichiometric ratios are needed: CsI (519.62 mg), SnI2 (745.038 mg), SnF2 (31.338 mg), Sn powder (1 mg), ascorbic acid (35.224 mg), and PMMA (1 mg) in the 1.25-mL DMSO solvent. The solution was stirred for 4 h at 1500 pm with 80 °C on the hotplate. The prepared CsSnI3 solution is shown in Fig. 1a, from which it can be found a very clear yellow color solution was formed.
a Clear transparent yellow color CsSnI3 solution before thin-film preparation. b An orange ring at the top of the CsSnI3 solution due to oxidation during the solution taking out for thin-film preparation. c The CsSnI3 solution becomes non-transparent, and the orange ring at the top of the solution goes away
Meanwhile, 2 mL of chlorobenzene and 2 mg of PFDD were put in another vial and then stirred for 1 h at 60 °C (Fig. 2a). After that, about 1 mg of CNTs was added to the chlorobenzene/PFDD mixed solution (Fig. 2b). Following that, the hybrid solution was sonicated in the bath sonicator for 1 h. During sonication, the temperature was set at 30 °C. From Fig. 2b, it is clear that the CNTs were dispersed very well in the solution, and a homogeneous and dark solution was obtained. Moreover, it was also found that the resultant solution remained stable; namely, the CNTs were dispersed very well even after 24 h, and there was no precipitation. Finally, 50 mL of CNT dispersed solution was added to the CsSnI3 solution. Then, the obtained mixed solution was further sonicated in the bath sonicator for 1 h. Notably, during sonication, the temperature of the bath sonicator was set at about 30 °C. Figure 2c shows the photo of the finally obtained CsSnI3/CNTs mixed precursor solution. After mixing the CNTs dispersed solution in the transparent yellow CsSnI3 solution, the mixed solution became a non-transparent orange solution. From Fig. 2c, it is seen that the polymer-wrapped CNTs were dispersed very well in the CsSnI3 solution. The excellent dispersion of the polymer-wrapped CNTs was one of the requirements for preparing the high-quality composite thin films because the CNTs could usually agglomerate into bundles during the preparation of the mixed precursor solution and spin-coating process due to the intra-tube van der Waals interactions within individual CNTs.
For thin-film preparation, some glass substrates, of which each had the size of 1.5 cm × 1.5 cm × 1 mm, were needed. The glass substrates were firstly washed in a bath sonicator with acetone, ethanol, and propanol, respectively, for 5 min each. At the time of sonication, the water temperature was set at about 30 °C. Then the substrates were heated in a vacuum oven at the temperature of 100 °C for about 10 min. The substrates were then dried with the flowing N2. After that, the substrates were further cleaned by the O2 plasma with 20% O2 for 5 min. As for the perovskite thin-film preparation, the spin coater was used. The vacuum of the spin coater was turned on to stick the substrate on the sample holder within the spin coater. For perovskite thin-film preparation, a 100-µL CsSnI3 solution (for the composite thin-film preparation, 100 µL of mixed CsSnI3/CNT solution) was dropped on the cleaned substrate. Thereafter, the substrate with the solution was spun at the speed of 1000 rpm for 5 s and subsequently 4000 rpm for 20 s. Then, the speed of the spin coater decreased gradually within 10 s. After that, various antisolvents with the amount of 400 µL were applied very slowly at the speed of about 2 drops per second. Finally, the obtained thin film was annealed at 100 °C for 5 min. All the procedures were done in ambient conditions, and the room temperature was about 30 °C, and the humidity was about 50%. Finally, the Cr electrodes were deposited on the CsSnI3 thin film by thermal evaporation.
As shown in Fig. 1, it can be observed that the oxidation of the CsSnI3 solution happened during thin-film preparation. Before using the perovskite solution for the thin-film preparation (see Fig. 1a), the color of the solution was transparent yellow. As shown in Fig. 1b, it was observed that an orange ring was formed on the top surface of the solution after making the solution open to the air. This orange ring was formed, which can be attributed to the oxidation of CsSnI3 solution while drawing the proper dose of precursor for the preparation of thin films. At that time, the solution came into contact with oxygen for a couple of seconds, as the cap of the vial was opened to take out the solution. It is interesting that, after a couple of minutes, the orange ring went away completely. But the solution then turned into non-transparent, as shown in Fig. 1c. It is predicted that this phenomenon that happened can be attributed to adding the extra Sn metal powder into the precursor solution.
The device structure and the related band structure of the all-inorganic perovskite photodetector are picturized in Fig. 3 (photodetector based on the CsSnI3/CNTs composite thin film and the related band structure are picturized in Fig. 4). As shown in Figs. 3a and 4a, the photodetector structure consists of a glass substrate, CsSnI3thin film or CsSnI3/CNTs composite thin film, and the Cr electrodes. As for a thin-film sample (glass substrate area was 1.5 × 1.5 cm2), there are a total of 20 devices in 4 rows and 5 columns. There are four different channel lengths of 50, 100, 150, and 200 µm, respectively, and all electrodes are with a length of 500 µm. The deposited Cr electrodes have a thickness of 200 nm. The band alignment of the fabricated photodetector devices is shown in Figs. 3b and 4b, where the conduction band (CB) and valence band (VB) of the all-inorganic perovskite, as well as the work function of the electrodes, can be obtained from the literature [63]. After applying light illumination, the photo-generated electrons and holes are separated. As the work function of the Cr electrodes is closer to the VB of CsSnI3 perovskite, it is expected that the photo-induced holes would be efficiently transferred to the Cr electrodes when the device is biased with an external source (see Fig. 3c), whereas the photo-generated electrons and holes efficiently transferred to the polymer-wrapped CNT from the CsSnI3 perovskite matrix, as depicted in Fig. 4c.
4 Thin films and relevant photodetector characterization
For the morphology characterization of specimen perovskite thin films, SEM (Zeiss Sigma VP HD) was used. For microanalysis of the perovskite thin-film samples, EDS (Integrated Oxford instrument AZtec EDS with X-Max 20 mm2 SDD EDS detector with Zeiss Sigma VP HD) was used. We performed AFM measurements for further morphology observation by using Bruker Dimension Icon Atomic Force Microscopy. XPS measurement was used for examining the chemical state of the perovskite thin film and here we used Thermo Fisher K-alpha XPS. XRD was used for identifying the crystalline phases of the perovskite thin film. In our experiment, we used PANalytical X’Pert Powder XRD. Here Raman spectroscopy was used for morphological and compositional characterization of the perovskite thin film. In our laboratory, we used Renishaw Raman inVia Reflex for Raman mapping measurements. Here 405-nm LASER was used to take the PL spectra. There was an option to change the intensity of LASER from 0 to 100. We have tuned the LASER intensity to 100 during the measurement. The absorption instrument was supplied from METASH. In this work, we tested the I-V curve of devices based on the perovskite thin films. For such measurements, we used a two-terminal probe station (EVERBEING INT’L CROP.) and 4200A-SCS PARAMETER ANALYZER. To measure the power of the applied light, we used optical power and an energy meter (PM100D, THORLABS). There are options for choosing specific wavelengths from 400 to 1600 nm. A white LED consists of a series of continuous wavelengths ranging from 400 to 700 nm. That is why the chosen wavelengths are within the range of 400–700 nm. All the above characterizations were done under ambient environmental conditions with open-air and normal room temperature.
5 Results and discussions
The morphology feature of the active perovskite films is crucial for achieving high-performance devices. Mainly, as for the Sn-based perovskite films, the low energy of formation of Sn vacancies results in high rates of defect-mediated charge carrier recombination and causes current leakage problems [33, 64]. Another challenge in achieving the high-quality solution-processed CsSnI3 thin film is how to get a low density of pinhole defects [65].
The SEM images of the CsSnI3 thin films deposited on glass substrates are shown in Fig. 6, from which it can be found there is a unique type of morphology formed in the perovskite films. The effects of the different anti-solvents on the morphology properties of the obtained films were also explored. In particular, different antisolvents such as toluene, ethyl acetate, and chlorobenzene were applied during the spin-coating process of the perovskite films. The chemical structures of different antisolvents (toluene, ethyl acetate, and chlorobenzene) are shown in Fig. 5. As for the CsSnI3 thin film that was prepared with toluene antisolvent, the relevant SEM images are shown in Fig. 6a and b, from which it can be found that the obtained perovskite film consists of lots of separate rectangular pillar-like structures. These pillar-like crystals have uniform grains with sizes larger than 1 µm. However, there are lots of holes formed within the perovskite film, leading to the lack of coverage of the thin film. The SEM images for perovskite thin film that was prepared with ethyl acetate antisolvent are shown in Fig. 6c and d. It can be found that there are lots of individual cylinder-like crystals. The average size of these cylinder-like crystals is less than 1 µm. Similar to the toluene antisolvent-treated perovskite film, it can also be observed that there are lots of holes within the thin film and a lack of surface coverage. Furthermore, the SEM images of the perovskite thin film prepared with the added chlorobenzene antisolvent are manifested in Fig. 6e and f, from which the resultant perovskite film is found to be with an equal distribution of rod-like crystals. The rod-like crystals are more uniform in size distribution, and the average size is about 1 µm. Moreover, it can be found that the thin film covered the whole surface of the substrate uniformly. From these SEM images, it can be seen that the CsSnI3 thin films with different antisolvent treatments can form other crystalline structures and surface morphologies. Among them, the chlorobenzene-treated perovskite film shows the best morphology feature. We got nano-sized rod-like crystalline morphology in the chlorobenzene-treated perovskite film, which would be favorable for fabricating the high-performance photodetector devices.
For getting further morphology details, the AFM measurements were performed on the surface of the chlorobenzene-treated CsSnI3 thin film. The obtained AFM images are shown in Fig. 7. We selected two different areas on the perovskite thin film and carried out the AFM tests with the exact scanning sizes, of which the obtained AFM images are shown in Fig. 7a and b, respectively. The AFM images can further confirm the nanorod-like crystals of the chlorobenzene-treated CsSnI3 thin film, from which it can be found the crystals’ average size is about 1 µm. This is consistent with results found from the SEM measurements, as shown in Fig. 6e and f. In addition, AFM images for the different areas of the same thin-film samples display similar morphological features, which further indicate that nanorod-like crystals can be distributed evenly in the whole thin film. The RMS roughness was found to be less than 15 nm within the 5 × 5 µm2 area, which demonstrates a quite uniform surface of the chlorobenzene-treated CsSnI3 thin film.
It should be noted that instability is still one of the big challenges for all kinds of halide perovskite materials, particularly the Sn-based perovskites like CsSnI3 thin films in this work, due to the susceptibility of Sn to be oxidized from the Sn2+ to the Sn4+ oxidation states upon exposure to air or moisture [22]. The surface of the CsSnI3 thin films would be more vulnerable to oxidation.
EDS map measurements were performed to provide an in-depth insight regarding the elemental components of the residuals on the surface of the obtained AIP thin films. Individual map analyses of each element for the chlorobenzene-treated CsSnI3 thin film are illustrated in Fig. 8a–d. The EDS mapping measurements were taken from a 400-µm2 area, and the depth of the EDS measurements was about 40% of the CsSnI3 thin film. From those figures, it is found that the compositional elements, such as Cs, Sn, and I, are evenly distributed on the surface of the prepared thin film. The mapping sum spectrum (see Fig. 8e) analysis indicates that the ratios among the elements Cs, Sn, and I are about 1:1:3. We also examined the oxidation of the surface of the thin film. It is observed that the whole surface of the thin film is oxidized; however, it should be noted that the map spectrum shows that the oxidation quantity is very low. The results from the EDS mapping indicate that the thin film was just oxidized at a low level. These results ensure that the CsSnI3 thin film that was prepared by adding chlorobenzene anti-solvent and PMMA is relatively stable in normal ambient open-air conditions.
The XPS measurements were also carried out to investigate the chemical states and to quantify the compositions of the surface for the chlorobenzene-treated CsSnI3 thin film. This analysis also helped with examining the surface oxidation of the CsSnI3 thin film as the surface oxidation of CsSnI3 directly affects its phase transformation, especially from the black orthorhombic phase to the non-perovskite yellow orthorhombic phase in the air [66]. From the XPS measurements, the characteristic peaks for Cs, Sn, and I elements of the perovskite thin film were analyzed, and the XPS survey spectrum can provide information on the concentrations of Cs, Sn, and I elements on the surface of the thin film. To determine the atomic concentrations, the following formula was used: \({x}_{1}=\left({y}_{1a}/{y}_{1b}\right)/\sum_{i}\left({y}_{ia}/{y}_{ib}\right)\), where x1 is the atomic fraction of element 1, y1a is the X-ray photoelectron intensity for element 1 in the matrix and yib is a sensitivity factor for element 1 in XPS, based on an average matrix [67]. It is found that the concentrations of Cs, Sn, and I are 4.34%, 15.94%, and 13.08%, respectively. Moreover, there was the presence of O on the surface of the perovskite thin film, and the concentration of O was found to be about 66.64%. Kumar et al. found Cs 3d core-level spectra, which is corresponding to Cs+ charge state at 726.04 eV and 739.92 eV for Cs 3d5/2 and Cs 3d3/2 peaks, respectively [33]; Sn4+ charge states at 485.6 eV and 494.1 eV, and Sn2+ at 485.2 and 493.7 eV, respectively, from the deconvoluted Sn 3d doublets; I− at 619.13 eV and 630.65 eV, and I3− at 620.03 eV and 631.51 eV at room temperature, respectively, from the deconvoluted two distinct oxidation states associated with I 3d doublets. According to XPS results from the research by Baranwal et al. [29], the Cs 3d spectra for CsSnI3 thin film showed 3d5/2 and 3d3/2 bands around 724.4 eV and 738.3 eV, respectively; spin–orbit doublet peaks of Sn 3d5/2 and Sn 3d3/2 bands are observed at 486.9 eV and 495.3 eV, respectively; I 3d spectra at around 618 eV and 632 eV for the spin–orbit doublet of I 3d5/2 and I 3d3/2. From Fig. 9a, it is found that Cs 3d5/2 and Cs 3d3/2 peaks are at 723.45 eV and 738.2 eV, respectively. According to Fig. 9b, Sn 3d5/2 and Sn 3d3/2 bands are found at 486.1 eV and 495.2 eV, respectively. The deconvoluted values of Sn 3d doublets: Sn4+ charge states at 486.2 eV and 494.9 eV, and Sn2+ at 486.7 and 494.1 eV, respectively. The spin–orbit doublet of I 3d5/2 and I 3d3/2 at 618.2 eV and 632.3 eV, respectively, is shown in Fig. 9c. The deconvoluted value of I 3d doublets: I− charge states at 617.7 eV and 632.1 eV and I3− at 618.1 eV and 628.4 eV. Marshall et al. surveyed the XPS measurements on prepared CsSnI3 thin films with the SnCl2 additives [15], and they found Sn2+ (486 eV, count 3500) and Sn4+ (486.80 eV, count 2300) for the fresh samples, and they turned to Sn2+ (486 eV, count 1100) and Sn4+ (487 eV, count 8500) after 1 h. They also found two weak O 1 s peaks at 531 eV and 532 eV, which can be assigned to SnO2 and H2O, respectively [68]. In the beginning, the count was 150 and 450, respectively, and after 1 h, the count was 4250 and 4600, respectively. Cao et al. added ascorbic acid into the CsSnI3 thin film and then examined the stability property [27]. They noted the Sn 3d peaks at 486.4 eV and 494.8 eV for the fresh ascorbic acid–treated CsSnI3 sample. They observed that, for the same ascorbic acid–treated CsSnI3 sample, the corresponding Sn 3d peaks could be remained at 486.2 eV and 494.7 eV after 1 day, suggesting the stability of the CsSnI3 sample. They also noted that for the CsSnI3 sample without the ascorbic acid treatment, the peak positions of Sn are changed to 487.1 eV and 495.5 eV, respectively, indicating that Sn2+ has been oxidized to Sn4+. Here, ascorbic acid is converted to glucose acid by reducing oxidized Sn4+ to Sn2+. In the chlorobenzene-treated CsSnI3 sample, Sn 3d spectra show at 486.1 eV and 495.2 eV after 2 days. The sample also shows Sn2+ (486.7 eV, count 300,000) and Sn4+ (486.2 eV, count 50,000) (Fig. 9b). The O 1 s peaks at 531 eV and 532.5 eV are also very low in count as shown in Fig. 9d. These results indicate that the sample is quite stable against oxidation in open-air condition.
XPS spectra of the chlorobenzene-treated CsSnI3 thin film. a Cs 3d5/2 at 723.45 eV and Cs 3d3/2 at 738.2 eV. b Sn 3d5/2 at 486.1 eV (Sn4+at 486.2 eV; Sn2+ at 486.7) and Sn 3d3/2 at 495.2 eV (Sn4+ at 494.9 eV; Sn2+ at 494.1 eV). c I 3d5/2 at 632.3 eV (I− at 632.1 eV; I3− at 628. 4 eV) and I 3d3/2 at 618.2 eV (I− at 617.7 eV; I3.− at 618.1 eV). d O 1 s at 531 eV and 532.5 eV
Just like other halide perovskites, the polycrystalline quality of CsSnI3 film can be tuned and controlled by changing the annealing temperatures. Usually, perovskite CsSnI3 can transform into the high-symmetry cubic (α) phase at high temperature, and the lower-symmetry phases of tetragonal (β) and orthorhombic (γ) can be formed upon the decreasing temperature due to tilting and distortions of the lattice [66, 69, 70]. The CsSnI3 normally exhibits two kinds of orthorhombic phases at room temperature. One has a yellow color (Y), while the other has a black color (B). When the orthorhombic B-γ-CsSnI3 is heated from room temperature to above 80 °C, it turns into the tetragonal B-β-CsSnI3 and then turns into the cubic B-α-CsSnI3 at above 150 °C in an inert atmosphere [71]. The yellow orthorhombic phase is the thermodynamically stable one at room temperature [66], while the orthorhombic black phase is a kinetically stabilized metastable phase [66]. It can transform to the yellow phase of Sn (IV) salt (Cs2SnI6) when prolonged exposure to air (oxygen or moisture) [72]. CsSnI3 thin-film samples are structurally characterized on the powder X-ray diffractometer at various exposure times to the ambient air. The color of the freshly prepared film was black, as can be seen in the inset of Fig. 10a. As shown in Fig. 10a, the XRD pattern for the fresh chlorobenzene-treated CsSnI3 thin-film sample displays all the peaks, consistent with the previous reports [15, 29, 63, 67, 72,73,74]. The results further confirm that the crystal structure corresponds to orthorhombic B-γ-CsSnI3. The characteristic peaks of CsSnI3 are found at the peaks of 12.6°, 14.4°, 20.5°, 22.9°, 24°, 25°, 27°, 29°, 31.7°, 32.7°, 35.9°, 41.3°, 41.8°, 37.3°, 43°, 44°, 45.5°, and 51.6°. Among them, the 14.4° and 25° peaks are highly intense, and the 29° peak is excessively high intense. Kumar et al. [67] assigned the B-γ-CsSnI3 crystal structure with a space group of Pnma (62), having the following lattice constants: a = 8.688 Å, b = 8.643 Å, c = 12.378 Å. They also observed that the prominently reflected planes of (131), (240), and (320) at 25.25°, 26.44°, and 27.61°, respectively, are the characteristics peaks of B-γ-CsSnI3. Baranwal et al. [29] also observed the same crystallographic pattern for B-γ-CsSnI3 and found two intense peaks at 14.43° and 29.10°, which corresponds to the planes of (101) and (202). Marshall et al. [15] used different tin-halide additives in the precursor solution, aiming to examine the stability of CsSnI3 thin film in an air environment, and they observed that, from the XRD evolution of the most vital (202) peak, the peak intensity did not change too much after about 1 day. Kontas et al. [72] grew CsSnI3 single crystal with the SnF2 additive, and they analyzed the structure of the single crystal and found there were two intense peaks at about 25° and 29°, which is corresponding to the phase of B-γ-CsSnI3. They also found that, after about 1 day, the two major peaks shifted towards 28° and 32°, and the sample shows the characteristics of Y-CsSnI3. They also found that, after 2 days, the two major peaks shifted towards 26.5° and 31°, and the sample turned into Cs2SnI6. Cao et al. [27] tested the stability of CsSnI3 thin film that was treated with ascorbic acid, and they observed that the major XRD peaks remained unchanged for up to 1 day; however, after 2 days, the film sample showed the XRD peaks that are related to Y-Cs2SnI6 in open-air conditions. In the case of my samples, after 1 day, the XRD pattern from the same sample (see Fig. 10b) still shows all the same characteristic peaks like those of the fresh samples, although the intensity of the peaks is lower than those of the fresh ones. Additionally, the black color of the thin film did not change. After 2 days, it was found that the XRD peaks with the lower intensity became much less intense, whereas the intensity of the main peaks also became weak (see Fig. 10c). The color of the thin film also faded a little bit. It is not expected that the mixed halide perovskite CsSnI3−xFx can be formed, due to the large radius difference between the two kinds of halide anions (F−: 2.2 Å; and I−: 1.33 Å). The characteristics peak of CsI is near 12.8° and that of SnI2 is near 27.6° 23. From our XRD pattern for the fresh CsSnI3 sample as shown in Fig. 10a, a very low intensity peak at 12.6° and a low intensity peak at 27° were found, which indicated that there were the few amount of residues of CsI and SnI2. Again, the 39.37° and 51.49° peaks can be attributed to the Cs2SnI6 phase [75]. In my sample, the 39.37° and 51.49° peaks were not found. This phenomenon can be probably attributed to the good stability property of my chlorobenzene-treated perovskite thin-film samples against the open-air ambient conditions.
The morphology property of the active semiconducting thin films, including halide perovskite materials, is crucial for achieving high-performance functional devices. Especially for Sn-based halide perovskite materials, the low energy for the formation of Sn vacancies can result in the high rates of defect-mediated charge carrier recombination, causing the significant current leakage problem [33, 64]. In addition, there is another challenge in achieving high-quality Sn-based perovskites, which is placed on how to get a low density of pinhole defects within the thin-film samples through the solution-processed growth methods [65]. We already demonstrated that the morphology properties of the all-inorganic perovskite CsSnI3 thin films could be tuned by using different antisolvents; in particular, the chlorobenzene-treated CsSnI3 films can have the most optimal morphology feature. In this research, we further explored the effect of the added semiconducting CNTs on the morphology characteristics of the CsSnI3-based composite thin films.
The SEM results of the chlorobenzene-treated pure CsSnI3 thin films and the CsSnI3/CNTs composite thin films deposited on the glass substrates are shown in Fig. 11a and b, respectively. It was found a nanorod-like nanocrystalline type of morphology in both cases. The films showed an equal distribution of nanorod-like crystals with uniform size distribution and almost regular in shape. The average size was about 1 µm in both cases. On the other hand, some anomalous-shaped discrete crystals for the CsSnI3/CNT composite are found in Fig. 11b. These anomalous-sized crystals formed might be because of a chemical change in the CsSnI3 solution. As to make a mixed solution, polymer-wrapped CNT dispersed chlorobenzene solution was added to the CsSnI3 precursor solution. Some of the anomalous-shaped crystals might be due to aggregation of CNTs, as it was too difficult to develop a well-distributed composite thin film. It was also quite challenging to identify the CNTs with SEM (Fig. 11b). So, we did Raman mapping to see the compositional distribution of the composite thin film.
The Raman mapping of the CsSnI3/CNTs composite thin film is depicted in Fig. 12. Spina et al. took the Raman spectra of spare CNTs and mixed MAPbI3/CNTs samples. They found no significant difference in the Raman D/G peak ratio between the two samples [76]. D and G peaks are two characteristic peaks of CNT Raman spectra. That means it is not possible to grasp the whole scenario of the presence of CNTs in composite thin films. That is why the Raman mapping technique should be the perfect way to verify the distribution of CNTs within the composite thin film. I carried out the Raman mapping measurements on the two different areas of the same composite thin film, of which the obtained images are displayed in Fig. 12. It is found that the CNTs could be evenly distributed in the selected area. From these features, it was further confirmed that the CNTs could be distributed evenly in the whole composite thin film. The results showed a nanonet-like network of CNTs within the composite thin film. This nanonet-like distribution of CNTs worked as connectors or electrodes between the nanorod-like CsSnI3 crystals. Sometimes they worked as a bypass of the CsSnI3 nanorod-like crystals.
The photoluminescence (PL) measurement was carried out at first. When excited with a 405-nm laser irradiation at room temperature, the B-γ-CsSnI3 exhibited an intense near-inferred (NIR) PL at around 950 nm (see Fig. 13a), which matches with the previous reports [63, 74]. The wavelength-dependent PL spectra of the pure CsSnI3 thin film is the violet one. The intense PL peak at around 950 nm under a weak photoexcitation indicates very high quantum efficiency, which is supportive of the direct bandgap nature of CsSnI3. The optical absorption spectra of the chlorobenzene-treated CsSnI3 thin film were also measured at room temperature. A typical absorption spectrum is displayed in Fig. 13b. The optical absorbance of the CsSnI3 thin film is shown in pink color. When the absorption spectrum measurements were performed, the absorption contribution from the glass substrate was removed at first. The absorption spectra clearly showed a sharp change at around 950 nm, which is similar to the cases in the previous reports [15, 63, 73, 74]. The value of the absorption coefficient steeply took off in the region of PL emission bandwidth and was zero below it. This obtained result certifies the direct bandgap nature of the CsSnI3 thin film. Based on the obtained absorption with the very sharp absorption edge, a direct bandgap of 1.3 eV can be calculated, which ensures that the obtained semiconductor sample is in the B-γ-CsSnI3 phase, in contrast to the case of possible obtained Cs2SnI6, which is a semiconductor with an indirect bandgap of 2.55 eV. The previous report showed that the absorption coefficient of Cs2SnI6 in the visible spectrum is about 10 times smaller than that of B-γ-CsSnI3 [77]. Overall, the obtained PL data and the favorable absorption properties further demonstrated the phase-pure AIP CsSnI3 thin films with high quality, making them very promising candidates for constructing high-performance photodetectors.
a PL spectra of the pure CsSnI3 thin film (violet color) and the CsSnI3/CNT composite thin film (cyan color) showing clearly the PL quenching as soon as the CNTs are added to form the hybrid materials. b UV–VIS absorption spectra of the pure CsSnI3 thin film (pink color) and the CsSnI3/CNT composite thin film (yellow color)
The wavelength-dependent PL spectra of the CsSnI3/CNTs composite thin film (the cyan one) are shown in Fig. 13a. As for the composite thin film, the PL intensity was found to be much lower than that of the pure CsSnI3 thin film, indicating the possible charge transfer between the CsSnI3 perovskite and the added CNTs within the composite thin film. Ka et al. [78] and Feng et al. [61] also reported similar PL results. We also analyzed the absorption properties of the pure CsSnI3 thin film and the CsSnI3/CNT composite thin film, of which the obtained absorption spectra covering the light wavelength range from the ultraviolet (UV) to the near-inferred (NIR) are shown in Fig. 13b. The optical absorbance of the CsSnI3/CNT composite thin film is shown in yellow color. The light absorption for the composite thin film took, such obtained result is similar to the cases in the other composites [60, 61, 79, 80]. The substantial enhancement of the absorption for the composite thin film should be attributed to the intense light absorption of the added CNTs [81]. The improvement of light absorption for the composite thin film is beneficial for improving the photosensitivity of the hybrid photodetector. This improved broadband UV–Vis-NIR light absorption can make the composite thin film an excellent light absorber, which holds promising potential for developing high-performance photodetectors.
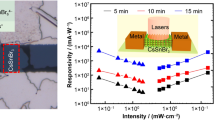
Due to the presence of intrinsic Sn vacancies (as stated in section 1), the electrical conductivity of B-α-CsSnI3 is high, and this high conductivity is demonstrated in other reports, which indicated that the high hole mobility is as high as 585 cm2V−1s−1[12, 66]. The conduction band (CB) and valence band (VB) of B-α-CsSnI3 were also determined to be at 3.62 and 4.92 eV, respectively [12]. Therefore, the Cr electrode is very suitable for the related photodetector because the work function of Cr is at 4.5 eV, which is situated just around the middle of the CB and VB of CsSnI3. Cao et al. [73] fabricated the photodetectors based on CsSnI3 thin films, for which the perovskite precursor solution was treated with extra SnI2 and ascorbic acid, and they found that the photocurrent for the obtained devices can be in the micron range. In this work, extra SnI2 and Sn powder have been used for preparing the precursor solution, which should reduce Sn vacancies, which can lower the conductivity properties of the obtained thin film, resulting in a lower dark current. Also, we added the ascorbic acid and PMMA for the preparation of the precursor solution, and these additives can provide a shield against oxidation and thus improve the thin films and the related device stability, as demonstrated by the above characterizations. As shown in Fig. 14a, the obtained photodetector showed a dark current in the range of 10−9 A, which can be attributed to the reduced conductivity properties of the perovskite films due to the reduced Sn vacancies. When applied, the white LED light illumination was onto the device, the obtained current was raised to the scale of up to 10−7 A (see Fig. 14b). However, it should be noted that such current value upon light radiation is about 10 times lower as compared to that obtained from the report by Cao et al. [73]. That means the reduced Sn vacancies within the perovskite thin films, in this case, can decrease not only the dark current but also the current under light illumination. Moreover, from Fig. 14b, there was only a weak hysteresis effect obtained in our device. It has been well-acknowledged that the hysteresis effect is highly related to the ion migration that has been widely observed in the halide perovskite materials, while the ion migration can be somehow linked to the vacancies within the halide perovskite materials. In particular, ion migration can take place mainly through the vacancies in the halide perovskites when applied to the biased field [82,83,84]. In this case, thanks to the reduced Sn vacancies in our chlorobenzene-treated perovskite thin film, the motion channels for the mobile ions would be reduced accordingly, thus leading to the weakened hysteresis behaviors. The photocurrent of the photodetectors based on the chlorobenzene-treated CsSnI3 thin films was measured under white LED light illumination. The power for different wavelengths is shown in the inset of Fig. 14c. The responsivity of the device was obtained by \(R=\frac{{I}_{ph}-{I}_{d}}{{P}_{\mathrm{light}}}\) where R is the responsivity, Iph is the photocurrent, Id is the dark current, and Plight is the incident light power. As the range of white light is 400–700 nm, I measured the power for different wavelengths within that range. As the white light cannot be separated from its monochromatic wavelengths, the responsivity for different wavelengths indicated the possible range. The responsivity of the devices seemed to be around the range of 10−5 A W−1. Recently, Han et al. [28] developed CsSnI3 nanowire array-based photodetector. The responsivity range of the photodetector was 10−3 A W−1 at 400–950 nm. Cao et al. [73] also developed a CsSnI3-based photodetector with ascorbic acid and SnF2 additives. Their photodetector also showed responsivity of the range of 10−1 A W−1 at 550–950 nm. The lower responsivity of my photodetector compared to others should be because of low photocurrent.
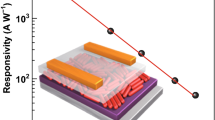
We further used the CsSnI3/CNT composite thin film as the active layer for fabricating the photodetectors with the Cr electrodes atop. Figure 15a schematically shows the planar structure of the fabricated photodetectors. Thereafter, we characterized the electrical properties of photodetectors based on the CsSnI3/SWCNT composite thin films in the dark and under light illumination, respectively, to evaluate the device performance. Fortunately, the particular morphology of the CsSnI3 thin film that we found was much more like lots of rod-like crystals whose average diameter was in the range of nanometers. The characteristics of rod-like crystals should be like nanorods. Therefore, the hybrid film mixed with polymer wrapped CNTs constituted a nanonet-like thin film. The CNTs offered fast carrier tracks between the nanorod-like crystals, and in some cases, the CNTs act like carrier tracks to overtake the nanorod-like crystals in the CsSnI3 thin film. The fundamental theme of the strategy is illustrated in Fig. 15b. After light illumination, the photo-generated electrons and holes efficiently transferred to the polymer wrapped CNT from the CsSnI3 perovskite matrix, as depicted in Fig. 15b. Here two interesting factors contributed to the improved performance. First, it was found that photo-generated charge carriers extracted quickly to CNTs. Second, the building blocks of CsSnI3 thin film, which were similar to nanorods, could be recognized as separated and independent unit devices. In this regard, the total thin-film resistance could be calculated to be RCsSnI3-1 + RCsSnI3-2 + RCsSnI3-3 + RCsSnI3-4 + ··· + RCsSnI3-N as shown in Fig. 15b. In the case of CNTs hybridized CsSnI3 thin film, when electrons faced to CNTs, the electrons pass over the CNTs ballistically due to the high conductivity of CNTs. The resistance of CNTs should be negligible compared to the CsSnI3. Therefore, the total resistance of the composite thin film was less than RCsSnI3-1 + RCsSnI3-2 + RCsSnI3-3 + RCsSnI3-4 + ··· + RCsSnI3-N and the new lower resistance value of composite thin film was RCsSnI3-1 + RCNT-1 + RCsSnI3-3 + RCsSnI3-4 + ··· + RCNT-N (see Fig. 15b). As CNTs were in the nanometer range and the nano rod-like crystals were in the micrometer range, the CNTs could be considered inside electrodes or collectors [85]. Thus, a faster response speed should be obtained due to reduced transit time and a low recombination rate. Such an efficient electron extraction will result in a fast response speed due to the efficient usage of excited electrons. Then, the responsivity and EQE should be improved, owing to the efficient charge extraction and transport.
a Schematic diagram of the fabricated planar photodetector with CsSnI3/CNT composite thin film as an active layer. b The photo-generated electrons face more resistance in the pristine CsSnI3 thin film than in the composite thin film. The bigger arrows (violet) refer to the ballistic transport of electrons
The CNTs were wrapped with the high bandgap PFDD. Electronic energy levels of this polymer lay well outside the bandgap of the CNTs, which corresponded to type I alignment. Thus, there should be no induce charge transfer between polymer/CNTs. Recent studies indicate that the wrapping polymer also does not hinder interfacial charge transfer [86, 87]. Hajnalka et al. [88] observed that upon visible light illumination, semiconducting CNT-based samples show photo-induced doping, originating from the charge carriers created in the perovskite. Incident photons produced electron–hole pairs in the CsSnI3 nanorod-like crystals. Holes were then transferred to the SWCNTs and led to the photocurrent. At the same time, electrons that remained in the CsSnI3 nanorod-like crystals faced a prolonged carrier lifetime and caused photodetection gain with capacitive coupling. Rachelle et al. [87] found rapid (picosecond) hole extraction from perovskite to CNTs and extremely slow back-transfer and recombination (microseconds) in highly enriched semiconducting CNTs/perovskite heterostructure films. The efficient hole extraction by the CNT layer also improved electron extraction by the electrodes, which should reduce charge accumulation at each critical interface and improve device efficiency and stability with reduced hysteresis. The dependence of the photo signal on the bias voltage is shown in Fig. 16a and b. It was found that the photocurrent depended linearly on the bias voltage, which indicates ohmic contact between thin film and electrodes. A clear change in current (from 10−6 A to 10−4 A) was noticed before and after illumination, indicating the on/off ratio of 102. The measured photoresponsivity is shown in Fig. 16c. As the white LED comprises wavelengths of around 400–700 nm, the specific wavelengths are chosen as 410, 440, 535, and 700 nm. With increasing wavelengths, the light power decreased. The photoresponsivity increased with decreasing light power. The photoresponsivity of the hybrid photodetector was in the range of about 10−2 A W−1. The responsivity is linearly related to the photocurrent. In the composite thin film, the total channel resistance was lower than the pure thin film. At the same time, the inclusion of CNTs reduced the possibility of photo-generated charge recombination and trappings. Thus, the photocurrent was increased. Therefore, the responsivity was increased about 1000 times compared to pure perovskite thin-film photodetector (responsivity of CsSnI3-based photodetector was 10−5 A W−1).
6 Conclusion
In this paper, we employed the all-inorganic Sn-based perovskite (CsSnI3) as the active layer for high-performance photodetectors. We demonstrated that the device performance could be further improved through morphology engineering and further via coupling with carbon nanotubes (CNTs). We first investigated the morphological engineering of CsSnI3 thin films with various antisolvent treatments. The morphological characterizations with SEM and AFM showed that the chlorobenzene-treated CsSnI3 thin film had nanorod-like nanocrystalline morphology. Moreover, the prepared CsSnI3 thin films with chlorobenzene treatment also showed excellent stability; namely, it was a pretty good oxidation resistance because it was found to be less oxidation through EDS and XPS characterizations. The CsSnI3 thin film also showed quite good stability in the ambient environment as it was found a more negligible effect on the crystalline peaks from XRD characterization. The PL curve showed a high-intensity peak at around 950 nm, confirmed from absorption spectra, as there was a sharp absorption change around that wavelength. Photodetectors made from the CsSnI3 thin films offered a dark current in the 10−9 A range. The photocurrent was about 100 times greater than the dark current and was 10−7 A. Thus, the on/off ratio was 102. The responsivity of the CsSnI3 thin-film photodetectors was 10−5 A W−1.
Furthermore, we also carried out the investigations on the photodetectors based on the all-inorganic perovskite (AIP) CsSnI3 thin films through coupling with the semiconducting CNTs. The morphological characterizations by SEM and Raman mapping techniques for the obtained CsSnI3/CNT composite thin films showed nanonet-like morphology. The PL spectra of the composite thin films manifested an efficient charge transfer effect between the CsSnI3 and the added CNTs within the composite films. Due to the addition of CNTs in the CsSnI3 thin film, the absorption of the composite film was also increased significantly. The photodetectors fabricated with the composite films offered a low dark current in the 10−6 A range and a high photocurrent in the 10−4 A range, thus the on/off ratio of 102. The responsivity of the hybrid photodetector was 10−2 A W−1.
References
M. Kulbak, D. Cahen, G. Hodes, How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 Cells. J. Phys. Chem. Lett. 6(13), 2452 (2015)
B.-W. Park, B. Philippe, X. Zhang, H. Rensmo, G. Boschloo, E.M.J. Johansson, Bismuth based hybrid perovskites A3Bi2I9 (A: methylammonium or cesium) for solar cell application. Adv. Mater. 27(43), 6806 (2015)
K. Eckhardt, V. Bon, J. Getzschmann, J. Grothe, F.M. Wisser, S. Kaskel, Crystallographic insights into (CH3NH3)3(Bi2I9): a new lead-free hybrid organic–inorganic material as a potential absorber for photovoltaics. Chem. Commun. 52(14), 3058 (2016)
M. Jahandar, J.H. Heo, C.E. Song, K.-J. Kong, W.S. Shin, J.-C. Lee, S.H. Im, S.-J. Moon, Highly efficient metal halide substituted CH3NH3I(PbI2)1−X(CuBr2)X planar perovskite solar cells. Nano Energy 27, 330 (2016)
T. Krishnamoorthy, H. Ding, C. Yan, W.L. Leong, T. Baikie, Z. Zhang, M. Sherburne, S. Li, M. Asta, N. Mathews, S.G. Mhaisalkar, Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 3(47), 23829 (2015)
K.M. Boopathi, P. Karuppuswamy, A. Singh, C. Hanmandlu, L. Lin, S.A. Abbas, C.C. Chang, P.C. Wang, G. Li, C.W. Chu, Solution-processable antimony-based light-absorbing materials beyond lead halide perovskites. J. Mater. Chem. A 5(39), 20843 (2017)
C. Zuo, L. Ding, Lead-free perovskite materials (NH4)3Sb2IxBr9–x. Angew. Chem. 129(23), 6628 (2017)
Z. Xiao, Z. Song, Y. Yan, From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 31(47), 1803792 (2019)
N.K. Noel, S.D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.-A. Haghighirad, A. Sadhanala, G.E. Eperon, S.K. Pathak, M.B. Johnston, A. Petrozza, L.M. Herz, H.J. Snaith, Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 7(9), 3061 (2014)
Z. Zhu, C.-C. Chueh, N. Li, C. Mao, A.K.-Y. Jen, Realizing efficient lead-free formamidinium tin triiodide perovskite solar cells via a sequential deposition route. Adv. Mater. 30(6), 1703800 (2018)
T.M. Koh, T. Krishnamoorthy, N. Yantara, C. Shi, W.L. Leong, P.P. Boix, A.C. Grimsdale, S.G. Mhaisalkar, N. Mathews, Formamidinium tin-based perovskite with low Eg for photovoltaic applications. J. Mater. Chem. A 3(29), 14996 (2015)
I. Chung, B. Lee, J. He, R.P.H. Chang, M.G. Kanatzidis, All-solid-state dye-sensitized solar cells with high efficiency. Nature 485(7399), 486 (2012)
I. Chung, J.-H. Song, J. Im, J. Androulakis, C.D. Malliakas, H. Li, A.J. Freeman, J.T. Kenney, M.G. Kanatzidis, CsSnI3: semiconductor or metal? High electrical conductivity and strong near-infrared photoluminescence from a single material. High Hole Mobility and Phase-Transitions. J. Am. Chem. Soc. 134(20), 8579 (2012)
N. Wang, Y. Zhou, M.-G. Ju, H.F. Garces, T. Ding, S. Pang, X.C. Zeng, N.P. Padture, X.W. Sun, Heterojunction-depleted lead-free perovskite solar cells with coarse-grained B-γ-CsSnI3 thin films. Adv. Energy Mater. 6(24), 1601130 (2016)
K.P. Marshall, M. Walker, R.I. Walton, R.A. Hatton, Enhanced stability and efficiency in hole-transport-layer-free CsSnI3 perovskite photovoltaics. Nat. Energy 1(12), 1 (2016)
J.H. Heo, J. Kim, H. Kim, S.H. Moon, S.H. Im, K.-H. Hong, Roles of SnX2 (X = F, Cl, Br) additives in tin-based halide perovskites toward highly efficient and stable lead-free perovskite solar cells. J. Phys. Chem. Lett. 9(20), 6024 (2018)
Y. Zhou, Y. Zhao, Chemical stability and instability of inorganic halide perovskites. Energy Environ. Sci. 12(5), 1495 (2019)
V.M. Goldschmidt, Die Gesetze der Krystallochemie. Naturwissenschaften 14(21), 477 (1926)
E.L. da Silva, J.M. Skelton, S.C. Parker, A. Walsh, Phase stability and transformations in the halide perovskite CsSnI3. Phys. Rev. B 91(14), 144107 (2015)
W. Travis, E.N.K. Glover, H. Bronstein, D.O. Scanlon, R.G. Palgrave, On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system. Chem. Sci. 7(7), 4548 (2016)
S. Tao, I. Schmidt, G. Brocks, J. Jiang, I. Tranca, K. Meerholz, S. Olthof, Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun. 10(1), 2560 (2019)
P.P. Boix, S. Agarwala, T.M. Koh, N. Mathews, S.G. Mhaisalkar, Perovskite solar cells: beyond methylammonium lead iodide. J. Phys. Chem. Lett. 6(5), 898 (2015)
P. Zhu, C. Chen, S. Gu, R. Lin, J. Zhu, CsSnI3 solar cells via an evaporation-assisted solution method. Solar RRL 2(4), 1700224 (2018)
A. Wijesekara, S. Varagnolo, G.D.M.R. Dabera, K.P. Marshall, H.J. Pereira, R.A. Hatton, Assessing the suitability of copper thiocyanate as a hole-transport layer in inverted CsSnI 3 perovskite photovoltaics. Sci. Rep. 8(1), 15722 (2018)
Y. Wang, J. Tu, T. Li, C. Tao, X. Deng, Z. Li, Convenient preparation of CsSnI3 quantum dots, excellent stability, and the highest performance of lead-free inorganic perovskite solar cells so far. J. Mater. Chem. A 7(13), 7683 (2019)
T. Zhang, H. Li, H. Ban, Q. Sun, Y. Shen, M. Wang, Efficient CsSnI3-based inorganic perovskite solar cells based on a mesoscopic metal oxide framework via incorporating a donor element. J. Mater. Chem. A 8(7), 4118 (2020)
F. Cao, W. Tian, M. Wang, M. Wang, L. Li, Stability enhancement of lead-free CsSnI3 perovskite photodetector with reductive ascorbic acid additive. InfoMat 2(3), 577 (2020)
M. Han, J. Sun, M. Peng, N. Han, Z. Chen, D. Liu, Y. Guo, S. Zhao, C. Shan, T. Xu, X. Hao, W. Hu, Z. Yang, Controllable growth of lead-free all-inorganic perovskite nanowire array with fast and stable near-infrared photodetection. J. Phys. Chem. C 123(28), 17566 (2019)
A.K. Baranwal, S. Saini, Z. Wang, D. Hirotani, T. Yabuki, S. Iikubo, K. Miyazaki, S. Hayase, Interface engineering using Y2O3 scaffold to enhance the thermoelectric performance of CsSnI3 thin film. Org. Electron. 76, 105488 (2020)
X. Xu, C.-C. Chueh, Z. Yang, A. Rajagopal, J. Xu, S.B. Jo, A.K.-Y. Jen, Ascorbic acid as an effective antioxidant additive to enhance the efficiency and stability of Pb/Sn-based binary perovskite solar cells. Nano Energy 34, 392 (2017)
H. Yao, F. Zhou, Z. Li, Z. Ci, L. Ding, Z. Jin, Strategies for improving the stability of tin-based perovskite (ASnX3) solar cells. Advanced Science 7(10), 1903540 (2020)
K.P. Marshall, R.I. Walton, R.A. Hatton, Tin perovskite/fullerene planar layer photovoltaics: improving the efficiency and stability of lead-free devices. J. Mater. Chem. A 3(21), 11631 (2015)
M.H. Kumar, S. Dharani, W.L. Leong, P.P. Boix, R.R. Prabhakar, T. Baikie, C. Shi, H. Ding, R. Ramesh, M. Asta, M. Graetzel, S.G. Mhaisalkar, N. Mathews, Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation. Adv. Mater. 26(41), 7122 (2014)
M. Xiao, S. Gu, P. Zhu, M. Tang, W. Zhu, R. Lin, C. Chen, W. Xu, T. Yu, J. Zhu, Tin-based perovskite with improved coverage and crystallinity through tin-fluoride-assisted heterogeneous nucleation. Advanced Optical Materials 6(1), 1700615 (2018)
W. Liao, D. Zhao, Y. Yu, C.R. Grice, C. Wang, A.J. Cimaroli, P. Schulz, W. Meng, K. Zhu, R.-G. Xiong, Y. Yan, Lead-free inverted planar formamidinium tin triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22%. Adv. Mater. 28(42), 9333 (2016)
Z. Zhao, F. Gu, Y. Li, W. Sun, S. Ye, H. Rao, Z. Liu, Z. Bian, C. Huang, Mixed-organic-cation tin iodide for lead-free perovskite solar cells with an efficiency of 812%. Adv. Sci. 4(11), 1700204 (2017)
L. Deng, K. Wang, H. Yang, H. Yu, B. Hu, Polymer assist crystallization and passivation for enhancements of open-circuit voltage and stability in tin-halide perovskite solar cells. J. Phys. D: Appl. Phys. 51(47), 475102 (2018)
X. Liu, K. Yan, D. Tan, X. Liang, H. Zhang, W. Huang, Solvent engineering improves efficiency of lead-free tin-based hybrid perovskite solar cells beyond 9%. ACS Energy Lett. 3(11), 2701 (2018)
J. Liang, J. Liu, Z. Jin, All-inorganic halide perovskites for optoelectronics: progress and prospects. Solar RRL 1(10), 1700086 (2017)
E.A. Duijnstee, J.M. Ball, V.M. Le Corre, L.J.A. Koster, H.J. Snaith, J. Lim, Toward understanding space-charge limited current measurements on metal halide perovskites. ACS Energy Lett. 5(2), 376 (2020)
J. Wei, Y. Zhao, H. Li, G. Li, J. Pan, D. Xu, Q. Zhao, D. Yu, Hysteresis analysis based on the ferroelectric effect in hybrid perovskite solar cells. J. Phys. Chem. Lett. 5(21), 3937 (2014)
D. Seol, A. Jeong, M.H. Han, S. Seo, T.S. Yoo, W.S. Choi, H.S. Jung, H. Shin, Y. Kim, origin of hysteresis in CH3NH3PbI3 perovskite thin films. Adv. Func. Mater. 27(37), 1701924 (2017)
S.P. Senanayak, B. Yang, T.H. Thomas, N. Giesbrecht, W. Huang, E. Gann, B. Nair, K. Goedel, S. Guha, X. Moya, C.R. McNeill, P. Docampo, A. Sadhanala, R.H. Friend, H. Sirringhaus, Understanding charge transport in lead iodide perovskite thin-film field-effect transistors. Sci. Adv. 3(1), e1601935 (2017)
S. Meloni, T. Moehl, W. Tress, M. Franckevičius, M. Saliba, Y.H. Lee, P. Gao, M.K. Nazeeruddin, S.M. Zakeeruddin, U. Rothlisberger, M. Graetzel, ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat. Commun. 7(1), 10334 (2016)
C. Li, S. Tscheuschner, F. Paulus, P.E. Hopkinson, J. Kießling, A. Köhler, Y. Vaynzof, S. Huettner, Iodine migration and its effect on hysteresis in perovskite solar cells. Adv. Mater. 28(12), 2446 (2016)
H. Lee, S. Gaiaschi, P. Chapon, A. Marronnier, H. Lee, J.-C. Vanel, D. Tondelier, J.-E. Bourée, Y. Bonnassieux, B. Geffroy, Direct experimental evidence of halide ionic migration under bias in CH3NH3PbI3–xClx-based perovskite solar cells using GD-OES analysis. ACS Energy Lett. 2(4), 943 (2017)
D.-Y. Son, S.-G. Kim, J.-Y. Seo, S.-H. Lee, H. Shin, D. Lee, N.-G. Park, Universal approach toward hysteresis-free perovskite solar cell via defect engineering. J. Am. Chem. Soc. 140(4), 1358 (2018)
D.W. Miller, G.E. Eperon, E.T. Roe, C.W. Warren, H.J. Snaith, M.C. Lonergan, Defect states in perovskite solar cells associated with hysteresis and performance. Appl. Phys. Lett. 109(15), 153902 (2016)
D.-Y. Son, J.-W. Lee, Y.J. Choi, I.-H. Jang, S. Lee, P.J. Yoo, H. Shin, N. Ahn, M. Choi, D. Kim, N.-G. Park, Self-formed grain boundary healing layer for highly efficient CH3NH3PbI3 perovskite solar cells. Nat. Energy 1(7), 1 (2016)
J.-W. Lee, S.-G. Kim, S.-H. Bae, D.-K. Lee, O. Lin, Y. Yang, N.-G. Park, The interplay between trap density and hysteresis in planar heterojunction perovskite solar cells. Nano Lett. 17(7), 4270 (2017)
Y. Lee, J. Kwon, E. Hwang, C.-H. Ra, W.J. Yoo, J.-H. Ahn, J.H. Park, J.H. Cho, High-performance perovskite–graphene hybrid photodetector. Adv. Mater. 27(1), 41 (2015)
Y. Wang, Y. Zhang, Y. Lu, W. Xu, H. Mu, C. Chen, H. Qiao, J. Song, S. Li, B. Sun, Y.-B. Cheng, Q. Bao, Hybrid graphene–perovskite phototransistors with ultrahigh responsivity and gain. Adv. Opt. Mater. 3(10), 1389 (2015)
P.-H. Chang, S.-Y. Liu, Y.-B. Lan, Y.-C. Tsai, X.-Q. You, C.-S. Li, K.-Y. Huang, A.-S. Chou, T.-C. Cheng, J.-K. Wang, C.-I. Wu, Ultrahigh responsivity and detectivity graphene–perovskite hybrid phototransistors by sequential vapor deposition. Sci. Rep. 7(1), 46281 (2017)
Z. Chen, Z. Kang, C. Rao, Y. Cheng, N. Liu, Z. Zhang, L. Li, Y. Gao, Improving performance of hybrid graphene–perovskite photodetector by a scratch channel. Advanced Electronic Materials 5(6), 1900168 (2019)
Y. Shao, Z. Xiao, C. Bi, Y. Yuan, J. Huang, Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 5(1), 5784 (2014)
J.-Y. Jeng, Y.-F. Chiang, M.-H. Lee, S.-R. Peng, T.-F. Guo, P. Chen, T.-C. Wen, CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 25(27), 3727 (2013)
L. Yang, S. Wang, Q. Zeng, Z. Zhang, L.-M. Peng, Carbon Nanotube photoelectronic and photovoltaic devices and their applications in infrared detection. Small 9(8), 1225 (2013)
S. Hong, S. Myung, A flexible approach to mobility. Nat. Nanotechnol. 2(4), 207 (2007)
W.-L. Xu, M.-S. Niu, X.-Y. Yang, J. Xiao, H.-C. Yuan, C. Xiong, X.-T. Hao, Carbon nanotubes as the effective charge transport pathways for planar perovskite photodetector. Org. Electron. 59, 156 (2018)
F. Li, H. Wang, D. Kufer, L. Liang, W. Yu, E. Alarousu, C. Ma, Y. Li, Z. Liu, C. Liu, N. Wei, F. Wang, L. Chen, O.F. Mohammed, A. Fratalocchi, X. Liu, G. Konstantatos, T. Wu, Ultrahigh carrier mobility achieved in photoresponsive hybrid perovskite films via coupling with single-walled carbon nanotubes. Adv. Mater. 29(16), 1602432 (2017)
F. Li, Z. Qiu, S. Liu, H. Zhang, Carbon nanotube-perovskite composites for ultrasensitive broadband photodiodes. ACS Appl. Nano Mater. 2(8), 4974 (2019)
R. Bandyopadhyaya, E. Nativ-Roth, O. Regev, R. Yerushalmi-Rozen, Stabilization of individual carbon nanotubes in aqueous solutions. Nano. Lett. 2(1), 25 (2002)
W.-L. Hong, Y.-C. Huang, C.-Y. Chang, Z.-C. Zhang, H.-R. Tsai, N.-Y. Chang, Y.-C. Chao, Efficient low-temperature solution-processed lead-free perovskite infrared light-emitting diodes. Adv. Mater. 28(36), 8029 (2016)
P. Xu, S. Chen, H.-J. Xiang, X.-G. Gong, S.-H. Wei, Influence of Defects and synthesis conditions on the photovoltaic performance of perovskite semiconductor CsSnI3. Chem. Mater. 26(20), 6068 (2014)
T. Yokoyama, D.H. Cao, C.C. Stoumpos, T.-B. Song, Y. Sato, S. Aramaki, M.G. Kanatzidis, Overcoming short-circuit in lead-free CH3NH3SnI3 perovskite solar cells via kinetically controlled gas–solid reaction film fabrication process. J. Phys. Chem. Lett. 7(5), 776 (2016)
I. Chung, J.-H. Song, J. Im, J. Androulakis, C.D. Malliakas, H. Li, A.J. Freeman, J.T. Kenney, M.G. Kanatzidis, CsSnI3: semiconductor or Metal? High electrical conductivity and strong near-infrared photoluminescence from a single material. High Hole Mobility and Phase-Transitions. J. Am. Chem. Soc. 134(20), 8579 (2012)
G.H.-J.S.K. Rajendra KumarKimKarupannanPrabakar, Interplay between iodide and tin vacancies in CsSnI3 perovskite solar cells. J. Phys. Chem. C 121(30), 16447 (2017)
P.R. Moses, L.M. Wier, J.C. Lennox, H.O. Finklea, J.R. Lenhard, R.W. Murray, X-ray photoelectron spectroscopy of alkylaminesilanes bound to metal oxide electrodes. Anal. Chem. 50(4), 576 (1978)
C.C. Stoumpos, C.D. Malliakas, M.G. Kanatzidis, Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52(15), 9019 (2013)
C.C. Stoumpos, L. Frazer, D.J. Clark, Y.S. Kim, S.H. Rhim, A.J. Freeman, J.B. Ketterson, J.I. Jang, M.G. Kanatzidis, Hybrid germanium iodide perovskite semiconductors: active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties. J. Am. Chem. Soc. 137(21), 6804 (2015)
K. Yamada, S. Funabiki, H. Horimoto, T. Matsui, T. Okuda, S. Ichiba, Structural phase transitions of the polymorphs of CsSnI3 by means of rietveld analysis of the X-ray diffraction. Chem. Lett. 20(5), 801 (1991)
A.G. Kontos, A. Kaltzoglou, E. Siranidi, D. Palles, G.K. Angeli, M.K. Arfanis, V. Psycharis, Y.S. Raptis, E.I. Kamitsos, P.N. Trikalitis, C.C. Stoumpos, M.G. Kanatzidis, P. Falaras, Structural stability, vibrational properties, and photoluminescence in CsSnI3 perovskite upon the addition of SnF2. Inorg. Chem. 56(1), 84 (2017)
F. Cao, W. Tian, M. Wang, M. Wang, L. Li, Stability enhancement of lead-free CsSnI3 perovskite photodetector with reductive ascorbic acid additive. InfoMat 2(3), 577 (2020)
K. Shum, Z. Chen, J. Qureshi, C. Yu, J.J. Wang, W. Pfenninger, N. Vockic, J. Midgley, J.T. Kenney, Synthesis and characterization of CsSnI3 thin films. Appl. Phys. Lett. 96(22), 221903 (2010)
X. Qiu, B. Cao, S. Yuan, X. Chen, Z. Qiu, Y. Jiang, Q. Ye, H. Wang, H. Zeng, J. Liu, M.G. Kanatzidis, From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48eV and high absorption coefficient. Solar Energy Mater. Solar Cells 159, 227 (2017)
M. Spina, B. Náfrádi, H.M. Tóháti, K. Kamarás, E. Bonvin, R. Gaal, L. Forró, E. Horváth, Ultrasensitive 1D field-effect phototransistors: CH3NH3PbI3 nanowire sensitized individual carbon nanotubes. Nanoscale 8(9), 4888 (2016)
J. Zhang, C. Yu, L. Wang, Y. Li, Y. Ren, K. Shum, Energy barrier at the N719-dye/CsSnI3 interface for photogenerated holes in dye-sensitized solar cells. Sci. Rep. 4(1), 6954 (2014)
I. Ka, L.F. Gerlein, I.M. Asuo, R. Nechache, S.G. Cloutier, An ultra-broadband perovskite-PbS quantum dot sensitized carbon nanotube photodetector. Nanoscale 10(19), 9044 (2018)
H. Zhu, A. Liu, H.L. Luque, H. Sun, D. Ji, Y.Y. Noh, Perovskite and conjugated polymer wrapped semiconducting carbon nanotube hybrid films for high-performance transistors and phototransistors. ACS Nano. 13(4), 3971 (2019)
W. Xu, Y. Guo, X. Zhang, L. Zheng, T. Zhu, D. Zhao, W. Hu, X. Gong, Room-temperature-operated ultrasensitive broadband photodetectors by perovskite incorporated with conjugated polymer and single-wall carbon nanotubes. Adv. Func. Mater. 28(7), 1705541 (2018)
P. Avouris, M. Freitag, V. Perebeinos, Carbon-nanotube photonics and optoelectronics. Nat. Photon. 2(6), 341 (2008)
T. Zhang, H. Chen, Y. Bai, S. Xiao, L. Zhu, C. Hu, Q. Xue, S. Yang, Understanding the relationship between ion migration and the anomalous hysteresis in high-efficiency perovskite solar cells: a fresh perspective from halide substitution. Nano Energy 26, 620 (2016)
P. Calado, A.M. Telford, D. Bryant, X. Li, J. Nelson, B.C. O’Regan, P.R.F. Barnes, Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 7(1), 1 (2016)
B. Chen, M. Yang, X. Zheng, C. Wu, W. Li, Y. Yan, J. Bisquert, G. Garcia-Belmonte, K. Zhu, S. Priya, Impact of capacitive effect and ion migration on the hysteretic behavior of perovskite solar cells. J. Phys. Chem. Lett. 6(23), 4693 (2015)
X. Li, D. Yu, J. Chen, Y. Wang, F. Cao, Y. Wei, Y. Wu, L. Wang, Y. Zhu, Z. Sun, J. Ji, Y. Shen, H. Sun, H. Zeng, Constructing fast carrier tracks into flexible perovskite photodetectors to greatly improve responsivity. ACS Nano. 11(2), 2015 (2017)
P. Schulz, A.-M. Dowgiallo, M. Yang, K. Zhu, J.L. Blackburn, J.J. Berry, Charge transfer dynamics between carbon nanotubes and hybrid organic metal halide perovskite films. J. Phys. Chem. Lett. 7(3), 418 (2016)
R. Ihly, A.-M. Dowgiallo, M. Yang, P. Schulz, N.J. Stanton, O.G. Reid, A.J. Ferguson, K. Zhu, J.J. Berry, J.L. Blackburn, Efficient charge extraction and slow recombination in organic–inorganic perovskites capped with semiconducting single-walled carbon nanotubes. Energy Environ. Sci. 9(4), 1439 (2016)
H.M. Tóháti, Á. Pekker, P. Andričević, L. Forró, B. Náfrádi, M. Kollár, E. Horváth, K. Kamarás, Optical detection of charge dynamics in CH3NH3PbI3/carbon nanotube composites. Nanoscale 9(45), 17781 (2017)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Ashraful Hossain Howlader independently completed this work involving searching for literature, carrying out the experiments, and manuscript drafting. Feng Li and Rongkun Zheng conceived and designed the project and revised and corrected the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Howlader, A.H., Li, F. & Zheng, R. Nanorod-like nanocrystalline CsSnI3 and CNT composite thin film–based hybrid photodetector. emergent mater. 5, 1925–1943 (2022). https://doi.org/10.1007/s42247-022-00394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-022-00394-8