Abstract
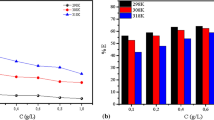
The corrosion inhibition performance of Olax subscorpioidea (OS) root extract on J55 carbon steel corrosion in acidizing solution stimulated with 15% HCl was studied using weight loss and electrochemical techniques as well as surface morphological study. The weight loss study showed a decrease in corrosion rate and an increase in inhibition efficiency by increasing the OS concentration. Potentiodynamic polarization measurements indicated that the OS extract is a mixed-type corrosion inhibitor but predominantly of anodic type. Electrochemical impedance spectroscopy (EIS) studies revealed that the introduction of OS in the 15% HCl solution increases the charge transfer resistance and simultaneously decreases the double-layer capacitance, enhancing an adsorbed film formation on the steel surface. Maximum inhibition efficiency of 89.7% was obtained with EIS study in the presence of 2.0 g/L of extract. The Fourier-transform infrared spectroscopy (FTIR) results indicated that the mechanism of inhibition is an absorption process through the functional groups present in OS molecules. The adsorption of OS onto the J55 steel surface followed the Langmuir adsorption model. Scanning electron microscopy (SEM), energy-dispersive X-ray spectra (EDS) analysis, and atomic force microscopy (AFM) confirm the formation of an adsorbed protective film of the OS molecules on the J55 steel surface. Quantum chemical calculations performed by density functional theory (DFT) on the major phytochemicals present in the OS root extract supported the experimental results and explained the adsorption behavior of the extracts.















Similar content being viewed by others
References
L.J. Kalfayan, Production enhancement with acid stimulation, 2nd edn. (PennWell, 2008)
C. Crowe, J. Masmonteil, R. Thomas, Oilfield Rev 4, 22 (1992)
Schlumberger, Reservoir stimulation, 3rd edn. (John Wiley & Sons, 2000)
M.H. Sliem, N.M. El Basiony, E.G. Zaki, M.A. Sharaf, A.M. Abdullah, Corrosion inhibition of mild steel in sulfuric acid by a newly synthesized Schiff base: an electrochemical, DFT, and Monte Carlo simulation study. Electroanalysis 32(12), 3145–3158 (2020)
N.B. Iroha, E.E. Oguzie, G.N. Onuoha, A.I. Onuchukwu, 16th Inter. Corros. Congr., Beijing, China, 126 (2005)
E. Berdimurodov, A. Kholikov, K. Akbarov, G. Xu, A.M. Abdullah, M. Hosseini, New anti-corrosion inhibitor (3ar,6ar)-3a,6a-di-p-tolyltetrahydroimidazo[4,5-d]imidazole-2,5(1 h,3h)-dithione for carbon steel in 1 M HCl medium: gravimetric, electrochemical, surface and quantum chemical analyses. Arab. J. Chem. 13(10), 7504–7523 (2020)
Y.L. Lv, F.Y. Kong, L. Zhou, Y.X. Hu, Q. Wang, Y.Q. Wang, X. Li, 1, 10-Phenanthroimidazole derivatives as efficient corrosion inhibitors for mild steel in 1 M HCl: synthesis, gravimetric, electrochemical and theoretical investigation. J. Mol. Struct. 129746 (2020). https://doi.org/10.1016/j.molstruc.2020.129746
G. Bahlakeh, B. Ramezanzadeh, A. Dehghani, M. Ramezanzadeh, Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: Detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 283, 174–195 (2019)
A. Saxena, D. Prasad, R. Haldhar, Investigation of corrosion inhibition effect and adsorption activities of Cuscuta reflexa extract for mild steel in 0.5 M H2SO4. Bioelectrochemistry 124, 156–164 (2018)
H. Gerengi, H.I. Sahin, Schinopsis lorentziiExtract As a Green Corrosion Inhibitor for Low Carbon Steel in 1 M HCl Solution. Ind. Eng. Chem. Res. 51, 780–787 (2012)
B. Ngouné, M. Pengou, A.M. Nouteza, C.P. Nanseu-Njiki, E. Ngameni, Performances of alkaloid extract from Rauvolfia macrophylla Stapf toward corrosion inhibition of C38 steel in acidic media. ACS Omega 4, 9081–9091 (2019)
A. Dehghani, G. Bahlakeh, B. Ramezanzadeh, A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 282, 366–384 (2019)
A.O. James, N.B. Iroha, IOSR J. Appl. Chem. 12(2), 01 (2019)
N.B. Iroha, N.J. Maduelosi, IOSR-JAC 13, 30 (2020)
O.K. Abiola, A.O. James, The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros. Sci. 52, 661–664 (2010)
E.E. Oguzie, C.K. Enenebeaku, C.O. Akalezi, S.C. Okoro, A.A. Ayuk, E.N. Ejike, Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J. Colloid Interface Sci. 349, 283–292 (2010)
N.B. Iroha, N.A. Madueke, Effect of Triumfetta rhomboidea leaves extract on the corrosion resistance of carbon steel in acidic environment. Chem. Sci. Int. J. 25, 1–9 (2018)
O.A. Adeoluwa, A.O. Aderibigbe, A.G. Drug Res. (Stuttg) 65, 306–311 (2015)
O.A. Adeoluwa, A.O. Aderibigbe, E.T. Olonode, Antinociceptive property of Olax subscorpioidea Oliv (Olacaceae) extract in mice. J. Ethnopharmacol. 156, 353–357 (2014)
I.T. Gbadamosi, L.A. Raji, A.A. Oyagbemi, T.O. Omobowale, Afr. J. Biomed. Res. 20, 293–299 (2017)
C. Verma, L.O. Olasunkanmi, I.B. Obot, E.E. Ebenso, M.A. Quraishi, 2,4-Diamino-5-(phenylthio)-5H-chromeno [2,3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 6, 53933–53948 (2016)
N.B. Iroha, N.A. Madueke, V. Mkpenie, B.T. Ogunyemi, L.B. Nnanna, S. Singh, E.D. Akpan, E.E. Ebenso, Experimental, adsorption, quantum chemical and molecular dynamics simulation studies on the corrosion inhibition performance of Vincamine on J55 steel in acidic medium. J. Mol. Struct. 129533 (2020). https://doi.org/10.1016/j.molstruc.2020.129533
N.B. Iroha, O. Akaranta, Experimental and surface morphological study of corrosion inhibition of N80 carbon steel in HCl stimulated acidizing solution using gum exudate from Terminalia Mentaly. SN Appl. Sci. 2, 1514 (2020)
K.F. Khaled, O.A. Elhabib, A. El-mghraby, O.B. Ibrahim, A.M. Magdy Ibrahim, J. Mater. Environ. Sci. 1, 139 (2010)
G. Achary, H.P. Sachin, Y.N. Arthola, T.V. Venkatesha, The corrosion inhibition of mild steel by 3-formyl-8-hydroxy quinoline in hydrochloric acid medium. Mater. Chem. Phys. 107, 44–50 (2008)
N.B. Iroha, L.A. Nnanna, J. Mater. Environ. Sci. 10, 898 (2019)
C. Verma, L.O. Olasunkanmi, I.B. Obot, E.E. Ebenso, M.A. Quraishi, 5-Arylpyrimido-[4,5-b]quinoline-diones as new and sustainable corrosion inhibitors for mild steel in 1 M HCl: a combined experimental and theoretical approach. RSC Adv. 6, 15639–15654 (2016)
N.B. Iroha, N.J. Maduelosi, Chem. Int. 6, 267 (2020)
J. Haque, V. Srivastava, C. Verma, H. Lgaz, R. Salghi, M.A. Quraishi, N-Methyl-N,N,N-trioctylammonium chloride as a novel and green corrosion inhibitor for mild steel in an acid chloride medium: electrochemical, DFT and MD studies. New J. Chem. 41, 13647–13662 (2017). https://doi.org/10.1039/c7nj02254a
N.J. Maduelosi, N.B. Iroha, Journal of Bio- and Tribo-Corrosion 7(6) (2021). https://doi.org/10.1007/s40735-020-00441-z
L. Tang, X. Li, Y. Si, G. Mu, G. Liu, The synergistic inhibition between 8-hydroxyquinoline and chloride ion for the corrosion of cold rolled steel in 0.5M sulfuric acid. Mater. Chem. Phys. 95, 29–38 (2006)
D.K. Yadav, M.A. Quraishi, Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind. Eng. Chem. Res. 51, 8194–8210 (2012)
D.S. Chauhan, M.A. Quraishi, A.A. Sorour, S.K. Saha, P. Banerjee, Triazole-modified chitosan: a biomacromolecule as a new environmentally benign corrosion inhibitor for carbon steel in a hydrochloric acid solution. RSC Adv. 9, 14990–15003 (2019)
N.B. Iroha, R.A. Ukpe, Communication in Physical Sciences. 5, 246–256 (2020)
G. Ji, P. Dwivedi, S. Sundaram, R. Prakash, Inhibitive effect of Chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 52, 10673–10681 (2013)
L. Larabi, Y. Harek, M. Traisnel, A. Mansri, Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1M HCl. J. Appl. Electrochem. 34, 833–839 (2004)
M. Elayyachy, A. El Idrissi, B. Hammouti, New thio-compounds as corrosion inhibitor for steel in 1M HCl. Corros. Sci. 48, 2470–2479 (2006)
A. Popova, M. Christov, Evaluation of impedance measurements on mild steel corrosion in acid media in the presence of heterocyclic compounds. Corros. Sci. 48, 3208–3221 (2006)
E. Naderi, A.H. Jafari, M. Ehteshamzadeh, M.G. Hosseini, Effect of carbon steel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1 M HCl solution by EIS. Mater. Chem. Phys. 115, 852–858 (2009)
K.F. Khaled, Experimental and computational investigations of corrosion and corrosion inhibition of iron in acid solutions. J. Appl. Electrochem. 41, 277–287 (2011)
P. Roy, P. Karfa, U. Adhikari, D. Sukul, Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: effect of intramolecular synergism. Corros. Sci. 88, 246–253 (2014)
C. Verma, A. Singh, G. Pallikonda, M. Chakravarty, M.A. Quraishi, I. Bahadur, E.E. Ebenso, Aryl sulfonamidomethylphosphonates as new class of green corrosion inhibitors for mild steel in 1M HCl: Electrochemical, surface and quantum chemical investigation. J. Mol. Liq. 209, 306–319 (2015)
V. Srivastava, D.S. Chauhan, P.G. Joshi, V. Maruthapandian, A.A. Sorour, M.A. Quraishi, Chemistry Select 3, 1990 (2018)
J. Haque, V. Srivastava, D.S. Chauhan, H. Lgaz, M.A. Quraishi, Microwave-induced synthesis of chitosan Schiff bases and their application as novel and green corrosion inhibitors: experimental and theoretical approach. ACS Omega 3, 5654–5668 (2018)
A.Y. El-Etre, Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 45, 2485–2495 (2003)
B. Ramaganthan, M. Gopiraman, L.O. Olasunkanmi, M.M. Kabanda, S. Yesudass, I. Bahadur, A.S. Adekunle, I.B. Obote, E.E. Ebenso, Synthesized photo-cross-linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies. RSC Adv. 5, 76675–76688 (2015)
P. Singh, E.E. Ebenso, L.O. Olasunkanmi, I. Obot, M. Quraishi, J. Phys. Chem. C 120, 3408–3419 (2016)
L. Herrag, B. Hammouti, S. Elkadiri, A. Aouniti, C. Jama, H. Vezin, Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 52, 3042–3051 (2010)
B. Xu, W. Yang, Y. Liu, X. Yin, W. Gong, Y. Chen, Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 78, 260–268 (2014)
N.V. Likhanova, M.A. Domínguez-Aguilar, O. Olivares-Xometl, N. Nava-Entzana, E. Arce, H. Dorantes, The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 52, 2088–2097 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
James, A.O., Iroha, N.B. New green inhibitor of Olax subscorpioidea root for J55 carbon steel corrosion in 15% HCl: theoretical, electrochemical, and surface morphological investigation. emergent mater. 5, 1119–1131 (2022). https://doi.org/10.1007/s42247-021-00161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-021-00161-1