Abstract
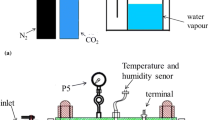
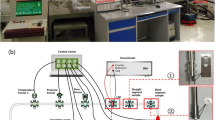
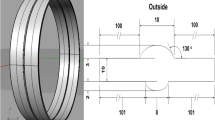
Corrosion failure accidents owing to flow erosion and pipeline corrosion frequently occur during transportation. The welding reinforcement height (WRH) can induce locally micro-turbulent flow field, which aggravates local corrosion of welded joints. A high wall shear stress (WSS) experimental setup was established to conduct the online electrochemical corrosion test. The influence of WRH sizes on local corrosion of welded joints was studied at different flow rates. The electrochemical signals of the local corrosion of X80 welded joints at different flow rates were monitored in real time using electrochemical impedance spectroscopy and wire beam microelectrode. In addition, the corrosion products composition and properties were analyzed. The results show that the micro-turbulent flow fields induced by the WRHs can enhance ion mass transfer near the welded joints. The corrosion products on the WRH surface also present different microscopic morphologies at different flow rates. In strong flow fields, the locally enhanced WSS can peel off the dense corrosion product partially, leading to the electrochemical distribution of large cathode and small anode, which accelerates the occurrence and development processes of the local corrosion of welded joints. The scientific guidelines for the corrosion protection of long-distance oil and gas pipelines can be potentially provided.



















Similar content being viewed by others
Abbreviations
- C :
-
Capacitor
- C 1 :
-
Actual concentration of Fe2+ at interface of corrosion product and solution
- \(C_{1\varepsilon }\) :
-
Constant, 1.42
- \(C_{2\varepsilon }\) :
-
Constant, 1.68
- \(C_{3\varepsilon }\) :
-
Constant related to directions of main stream and gravity
- C b :
-
Concentration of Fe2+ in main solution
- \(C_{{{\text{b}},{\text{H}}^{ + } }}\) :
-
\({\text{H}}^{ + }\) concentration in main solution
- C e :
-
Constants in stable solution system
- C 0 :
-
Actual concentration of FeCO3 at junction of metal and corrosion product
- \(C_{{{\text{s}},{\text{H}}^{ + } }}\) :
-
\({\text{H}}^{ + }\) concentration in reaction interface
- \(C_{\mu }\) :
-
Constant, 0.0845
- \(d\) :
-
Constant, 0.5
- \(D_{{{\text{H}}^{ + } }}\) :
-
\({\text{H}}^{ + }\) diffusion coefficient
- \(D_{{{\text{Fe}}^{2 + } }}\) :
-
Diffusion coefficient of corrosion product
- \(F\) :
-
Faraday constant
- \({i}_{{\text{lim}},{{\text{H}^{+}}}}\) :
-
Limit current density
- \(g\) :
-
Gravitational acceleration, 9.8 m/s2
- \(G_{k}\) :
-
Turbulent kinetic energy due to average velocity gradient, m/s2
- \(G_{{\text{b}}}\) :
-
Turbulent kinetic energy due to buoyancy, m/s2
- \(k\) :
-
Turbulent kinetic energy, m/s2
- \(k_{{{\text{H}}^{ + } }}\) :
-
Mass transfer coefficient
- \(K_{{\text{r}}}\) :
-
Corrosion rate constant
- \(L\) :
-
Inductance, H cm2
- \(l\) :
-
Characteristic length
- \(n\) :
-
Coefficient of dispersion
- \(n_{\text{dl}}\) :
-
Coefficient dispersion of double-layer capacitor
- \(n_{\text{pf}}\) :
-
Coefficient dispersion of corrosion product
- \(p\) :
-
Pressure, Pa
- \(Q_{{{\text{dl}}}}\) :
-
Constant phase element of double-layer capacitor, μF/cm2
- \(Q_{{{\text{pf}}}}\) :
-
Constant phase element of corrosion product, μF/cm2
- \(R\) :
-
Radius of welding reinforcement height region, mm
- \(R_{{{\text{ct}}}}\) :
-
Charge transfer resistance, Ω cm2
- Re :
-
Reynolds number
- \(R_{{\text{L}}}\) :
-
Inductance resistance, Ω cm2
- \(R_{{{\text{pf}}}}\) :
-
Corrosion product resistance, Ω cm2
- \(R_{{\text{s}}}\) :
-
Solution resistance, Ω cm2
- \(R_{\varepsilon }\) :
-
Correction term
- Sc :
-
Schmidt number
- Sh :
-
Sherwood number
- \(S_{k}\) :
-
User-defined source term
- \(S_{{\text{p}}}\) :
-
Particle interaction on fluid, Pa
- \(S_{\varepsilon }\) :
-
User-defined source term
- \(T\) :
-
Solution temperature, °C
- \(t\) :
-
Time, h
- \(u\) :
-
Liquid velocity, m/s
- \(\vec{u}\) :
-
Velocity vector
- \(u_{i}\) :
-
Velocity in i direction
- \(x_{i}\) :
-
x coordinate component
- \(x_{j}\) :
-
x coordinate component
- \(Z^{\prime}\) :
-
Real part of impedance
- \(Z^{\prime\prime}\) :
-
Imaginary part of impedance
- \(\left| Z \right|\) :
-
Impedance magnitude
- \(\alpha_{k}\) :
-
Effective Prandtl number of reciprocal of k, 1.39
- \(\alpha_{\varepsilon }\) :
-
Effective Prandtl number of reciprocal of \(\varepsilon\), 1.39
- β :
-
Constant, 0.012
- \(\delta\) :
-
Deposited corrosion product thickness
- \(\delta_{{\text{l}}}\) :
-
Thickness of effective concentration boundary layer
- \(\varepsilon\) :
-
Turbulent dissipation rate, m2/s3
- η :
-
User-defined source term
- \(\eta_{0}\) :
-
Constant term, 4.38
- \(\theta\) :
-
Porosity
- μ :
-
Solution dynamic viscosity, Pa s
- \(\mu_{{{\text{eff}}}}\) :
-
Effective kinematic viscosity of fluid, Pa s
- ν :
-
Solution kinematic viscosity, Pa s
- \(\rho\) :
-
Liquid density, kg/m3
- \(\overline{\tau }\) :
-
Stress tensor, Pa
References
Z. Tan, L. Yang, D. Zhang, Z. Wang, F. Cheng, M. Zhang, Y. Jin, J. Mater. Sci. Technol. 49 (2020) 186–201.
Z. Tang, D. Zhang, L. Yang, Z. Wang, F. Cheng, M. Zhang, Y. Jin, S. Zhu, Tribol. Int. 146 (2020) 106145.
Z.B. Wang, Y.G. Zheng, J. Pipeline Sci. Eng. 1 (2021) 63–73.
S.T. Kim, I.S. Lee, J.S. Kim, S.H. Jang, Y.S. Park, K.T. Kim, Y.S. Kim, Corros. Sci. 64 (2012) 164–173.
X. Dou, Z. He, X. Zhang, Y. Liu, R. Liu, Z. Tan, D. Zhang, Y. Li, Colloids Surf. A 665 (2023) 131225.
B.O. Okonkwo, H. Ming, Z. Zhang, J. Wang, E. Rahimi, S. Hosseinpour, A. Davoodi, Corros. Sci. 154 (2019) 49–60.
R. Liu, Y. Liu, D. Zhang, Frontiers Mater. 9 (2022) 910319.
D. Zhang, L. Yang, Z. Tan, S. Xing, S. Bai, E. Wei, X. Tang, Y. Jin, Experiment. Therm. Fluid Sci. 124 (2021) 110333.
L. Yang, D. Zhang, H. Fan, Z. Tan, S. Xing, X. Guan, X. Jiang, J. Nat. Gas Sci. Eng. 106 (2022) 104745.
K. Avila, D. Moxey, A. de Lozar, M. Avila, D. Barkley, B. Hof, Science 333 (2011) 192–196.
J.H. Kim, I.S. Kim, H.S. Chung, Canad. Metall. Q. 42 (2013) 253–260.
R.G. Kelly, J.R. Scully, D. Shoesmith, R.G. Buchheit, The Influence of Mass Transport on Electrochemical Processes, CRC Press, Boca Raton, USA, 2002.
Y. Li, J. Li, K. Wang, L. Song, C. Du, R. He, J. Mater. Eng. Perform. 27 (2018) 6636–6647.
G.A. Zhang, D. Liu, Y.Z. Li, X.P. Guo, Corros. Sci. 120 (2017) 107–120.
S. Nesic, Energy Fuels 26 (2012) 4098–4111.
S. Nešić, G.T. Solvi, J. Enerhaug, Corrosion 51 (1995) 773–787.
G. Zhang, L. Zeng, H. Huang, X. Guo, Corros. Sci. 77 (2013) 334–341.
L. Wei, X. Pang, K. Gao, Corros. Sci. 136 (2018) 339–351.
M. Javidi, S. Bekhrad, Eng. Fail. Anal. 89 (2018) 46–56.
J.L. Mora-Mendoza, S. Turgoose, Corros. Sci. 44 (2002) 1223–1246.
J. Zhang, Z.L. Wang, Z.M. Wang, X. Han, Corros. Sci. 65 (2012) 397–404.
M. Gao, X. Pang, K. Gao, Corros. Sci. 53 (2011) 557–568.
W. Li, B. Pots, X. Zhong, S. Nešić, Corros. Sci. 126 (2017) 208–226.
Z.W. Tan, Z.B. Wang, S.Y. Bai, D.L. Zhang, S.Z. Zhang, F. Cheng, S.H. Xing, Y.H. Jin, J. Iron Steel Res. Int. 29 (2022) 1026–1038.
A. Li, Z. Wang, L. Zhu, Z. Wang, J. Shi, W. Yang, Particuology 63 (2022) 83–94.
E.U. Hartge, L. Ratschow, R. Wischnewski, J. Werther, Particuology 7 (2009) 283–296.
Y. Xu, Q. Zhang, H. Chen, Y. Huang, J. Mater. Res. Technol. 25 (2023) 6550–6566.
L. Li, Y. Qiao, L. Zhang, A. Ma, E.F. Daniel, R. Ma, J. Chen, Y. Zheng, Int. J. Miner. Metall. Mater. 30 (2023) 1338–1352.
Li, L, Y.X. Qiao, L.M. Zhang, C.T. Li, Z. Liu, R.Y. Ma, L.L. Yang, J.Y. Li, Y.G. Zheng, Ultrason. Sonochem. 98 (2023) 106498.
Q.Y. Liu, L.J. Mao, S.W. Zhou, Corros. Sci. 84 (2014) 165–171.
H.J. Kim, K.H. Kim, Nucl. Eng. Des. 301 (2016) 183–188.
G.A. Zhang, M.X. Lu, Y.B. Qiu, X.P. Guo, Z.Y. Chen, J. Electrochem. Soc. 159 (2012) C393–C402.
F. Farelas, M. Galicia, B. Brown, S. Nesic, H. Castaneda, Corros. Sci. 52 (2010) 509–517.
A. Kahyarian, S. Nesic, J. Electrochem. Soc. 166 (2019) C3048–C3063.
Z.Y. Hu, D.L. Duan, S.H. Hou, X.J. Ding, S. Li, J. Mater. Sci. Technol. 31 (2015) 1274–1281.
G.A. Zhang, Y.F. Cheng, Corros. Sci. 51 (2009) 87–94.
D. Zheng, D. Che, Y. Liu, Corros. Sci. 50 (2008) 3005–3020.
R. Ghosh, M. Chinara, K. Godbole, K. Mondal, S. Mukherjee, J. Mater. Eng. Perform. (2023) https://doi.org/10.21203/rs.3.rs-2482803/v1.
A. Kahyarian, M. Singer, S. Nesic, J. Nat. Gas Sci. Eng. 29 (2016) 530–549.
F.A. Abdulaleem, F.M. Al Habdan, M.E. El Dahshan, Key Eng. Mater. 20–28 (1991) 383–396.
B. Poulson, Corros. Sci. 30 (1990) 743–746.
M. Prasad, V. Gopika, A. Sridharan, S. Parida, Prog. Nucl. Energy 107 (2018) 205–214.
L.E. Sanchez-Caldera, P. Griffith, E. Rabinowicz, J. Eng. Gas Turbines Power 110 (1988) 180–184.
N.Y. Lee, S.G. Lee, K.H. Ryu, I.S. Hwang, Nucl. Eng. Des. 237 (2007) 761–767.
D.P. Wang, Characterization of the local mass transfer rate downstream of an orifice, Mc Master University, Hamilton, Canada, 2012.
Acknowledgements
This work was conducted with financial support from the National Natural Science Foundation of China (Nos. 52206199, 42176209, 51979282, and 41676071) and the Natural Science Foundation of Shandong Province (No. ZR2021MD064).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dou, Xh., Li, B., He, Zh. et al. CO2 corrosion of X80 steel welded joints under micro-turbulence induced by welding reinforcement height. J. Iron Steel Res. Int. 31, 1015–1032 (2024). https://doi.org/10.1007/s42243-023-01091-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-023-01091-4