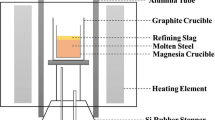
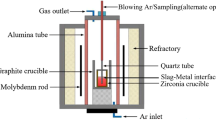
Abstract
A thermodynamic model for predicting the equilibrium oxygens of 304 stainless steel was developed based on the theory of slag–steel equilibrium, the law of mass conservation, and the ion and molecule coexistence theory. In the developed model, the Fe–Cr–Mn–Si–Al–S–O–melts reaction system and CaO–MgO–CaF2–FeO–MnO–Al2O3–SiO2–Cr2O3 slags were considered. The oxygen contents calculated by the model are in good agreement with experimental results and reference data. The equilibrium oxygen contents in 304 stainless steel mainly decrease with increasing binary basicity (\(w_{{({\text{CaO}})}} /w_{{({\text{SiO}}_{2} )}}\), where w(i) is the mass percentage of component i) and decreasing temperature. Controlling binary basicity at 2.0 while maintaining temperatures lower than 1823 K will keep the oxygen contents in the 304 stainless steel lower than 15 × 10–6. The equilibrium oxygen contents may also be decreased with increasing content of MgO in slags, which is more significant at lower binary basicity. Besides, a small amount of FeO, MnO, and Al2O3 (about 0–2.5 wt.%) in slags has little effect on equilibrium oxygen contents. Furthermore, it is found that the [C]–[O] reaction may occur during refining process but will not significantly affect the equilibrium oxygen contents.












Similar content being viewed by others
Abbreviations
- \(a_{(i)}^{{\text{e}}}\) :
-
Activity of component i in equilibrated slag
- \(a_{[i]}^{{\text{e}}}\) :
-
Activity of component i in equilibrated steel
- \(B\) :
-
Binary basicity (w(CaO)/\(w_{(\text{SiO}_2)}\)) of CaO–MgO–CaF2–FeO–MnO–Al2O3–SiO2–Cr2O3 slags
- \(e_{i}^{j}\) :
-
Activity interaction coefficient of component j on component i in molten steel based on mass percentage as concentration unit and 1 mass percent (1 wt.%) as standard state
- \(f_{i}^{{\text{e}}}\) :
-
Activity coefficient of component i in equilibrated metal phase based on mass percentage as concentration unit and 1 mass percent (1 wt.%) as a standard state
- \(\Delta_{\text{r}}{{G}}_{\text{m}}^{\ominus} \) :
-
Standard molar Gibbs free energy
- \(\Delta_{{\text{r}}} G_{{{\text{m}},[{\text{M}}] - [{\text{O}}]}}^{ \ominus }\) (\(\Delta_{{\text{r}}} G_{{{\text{m}},({\text{MS)}} - [{\text{O}}]}}^{ \ominus }\)):
-
Standard molar Gibbs free energy changes of [M]–[O] ((MS)–[O]) reaction, J mol–1
- \(\Delta_{{\text{r}}} G_{{{\text{m}},[{\text{M}}] - [{\text{O}}]}}^{{}}\) :
-
Molar Gibbs free energy changes of [M]–[O] reaction, J mol–1
- \(K_{{[{\text{M}}] - [{\text{O}}]}}^{ \ominus }\) (\(K_{{({\text{O}}) - [{\text{MS}}]}}^{ \ominus }\)):
-
Equilibrium constant of [M]–[O] ((O)–[MS]) reaction
- \({{K_{{\text{c}}i}}}\) :
-
Equilibrium constant of a complex molecular formation reaction equation code-named ci
- \(M_{i}\) :
-
Molecular mass of element i or component i, g mol–1
- \({\text{MO}}_{x}\) (\({\text{MO}}\)):
-
Oxide component in slags
- \(N_{i}\) :
-
Mass action concentrations of structural unit i or ion couple i in equilibrated slags based on IMCT
- \(n_{i}^{{\text{e}}}\) :
-
Mole amount of components i in equilibrated slag, mol
- \(n_{i}\) :
-
Mole amount of structural unit i, or ion couple i in equilibrated slag, mol
- \(\sum {n_{i}^{{}} }\) :
-
Sum of mole amounts of all structural units and ion couples in equilibrated slags, mol
- \(\Delta n_{{[{\text{O}}], \, [{\text{M}}] - [{\text{O}}]}}\) :
-
Molar change of oxygen in molten steel caused by [M]–[O] reaction over whole course of reaction, mol
- \(\sum {\Delta n_{{[{\text{O}}], \, [{\text{M}}] - [{\text{O}}]}} }\) :
-
Total molar change of oxygen in molten steel over whole course of reaction, mol
- \(p_{{{\text{CO}}}}\) :
-
CO partial pressure in furnace, Pa
- \(R\) :
-
Gas constant, 8.314 × 103 kJ mol–1 K–1
- \(T\) :
-
Absolute temperature, K
- \(W_{{[{\text{M}}]}}^{{\text{e}}}\) (\(W_{{[{\text{M}}]}}^{{\text{i}}}\)):
-
Mass of element M in equilibrated (initial) metal phase, g
- \(W_{{({\text{M}})}}^{{\text{e}}}\) (\(W_{{({\text{M}})}}^{{\text{i}}}\)):
-
Mass of element M in equilibrated (initial) slag phase, g
- \(W_{{{\text{metal}}}}^{{\text{e}}}\) (\(W_{{{\text{metal}}}}^{{\text{i}}}\)):
-
Mass of equilibrated (initial) metal, g
- \(W_{{{\text{slag}}}}^{{\text{e}}}\) (\(W_{{{\text{slag}}}}^{{\text{i}}}\)):
-
Mass of equilibrated (initial) slag, g
- \(W_{{{\text{slag}}}}\) :
-
Mass of slag phase
- \(W_{{{\text{metal}}}}\) :
-
Mass of metal phase
- \(\Delta W_{{[{\text{C}}]}}\) :
-
Mass reduction of C element in 100 g steel during whole process of slag–metal reactions (\(\Delta W_{{[{\text{C}}]}} = W_{{[{\text{C}}]}}^{{\text{i}}} - W_{{[{\text{C}}]}}^{{\text{e}}}\)), g
- \(w_{(i)}\) :
-
Mass percentage of component i
- \(w_{(i)}^{{\text{e}}}\) (\(w_{(i)}^{{\text{i}}}\)):
-
Mass percentage of component i in equilibrated (initial) slag phase, wt.%
- \(w_{[i]}^{{\text{e}}}\) (\(w_{[i]}^{{\text{i}}}\)):
-
Mass percentage of component i in equilibrated (initial) metal phase, wt.%
- \(w_{{[{\text{O}}],{\text{Calculated}}}}^{{{\text{e}},{\text{ Coupled}}}}\) :
-
Equilibrium oxygen content calculated by developed model, wt.%
- \(w_{{[{\text{O}}],{\text{Calculated}}}}^{{{\text{e}},{\text{ [M]}} - {\text{[O]}}}}\) :
-
Equilibrium oxygen content calculated by [M]–[O] reaction
- \(w_{{[{\text{O}}],{\text{Measured}}}}^{{\text{e}}}\) :
-
Measured oxygen content in final steel, wt.%
- \(w_{{[{\text{O}}],{\text{Calculated}}}}^{{{\text{e}},{\text{ Coupled with [C]}} - {\text{[O]}}}}\) :
-
Equilibrium oxygen content calculated by established model considering [C]–[O] reaction, wt.%
- \(x\) :
-
Stoichiometric numbers of M and O elements in oxide MOx
- []:
-
Component in metal phase
- ():
-
Component in slag
- metal:
-
Metal phase
- slag:
-
Slag phase
- \([{\text{M}}]\!\! -\! \![{\text{O}}]\) :
-
Interface reaction \(x[{\text{M}}] + y[{\text{O}}] = ({\text{M}}_{x} {\text{O}}_{y} )\)
- \({\text{(MS)}}\!\! -\! \![{\text{O}}]\) :
-
Interface reaction \(\left( {{\text{MS}}} \right) + \left[ {\text{O}} \right] = \left( {{\text{MO}}} \right) + \left[ {\text{S}} \right]\)
- e:
-
Equilibrium state
- i:
-
Initial state
References
S.Q. Zheng, C.Y. Li, Y.M. Qi, L.Q. Chen, C.F. Chen, Corros. Sci. 67 (2013) 20–31.
L.Z. Wang, Y.T. Li, S.F. Yang, J.Q. Li, C.Y. Chen, C.R. Li, B.Y. Tuo, Metall. Mater. Trans. B 53 (2022) 1212–1223.
E.N. Moran, G.S. Frankel, Y.H. Kim, Corrosion 67 (2011) 95005.
M.A. Baker, J.E. Castle, Corros. Sci. 33 (1992) 1295–1312.
P.C. Yan, S.G. Huang, L. Pandelaers, J. Van Dyck, M.X. Guo, B. Blanpain, Metall. Mater. Trans. B 44 (2013) 1105–1119.
C.H. Jiang, D. Tang, C. Zhang, Y. Zhang, A.M. Zhao, Mater. Res. Innov. 18 (2014) S4-281.
L.F. Zhang, B.G. Thomas, ISIJ Int. 43 (2003) 271–291.
Y. Ren, L.F. Zhang, S.S. Li, ISIJ Int. 54 (2014) 2772–2779.
J. Jun, K. Holguin, G.S. Frankel, Corrosion 70 (2014) 146–155.
J. Guo, S.S. Cheng, Z.J. Cheng, J. Iron Steel Res. Int. 21 (2014) 166–173.
K. Mineura, I. Takahash, K. Tanaka, ISIJ Int. 30 (1990) 192–198.
S.Y. Li, B. Li, X.M. Zhao, X.J. Xi, S.C. Duan, J. Guo, H. Guo, J. Iron Steel Res. Int. 28 (2021) 978–989.
S.Y. Li, B. Li, S.C. Duan, X.M. Zhao, J. Guo, H.J. Guo, J. Cent. South Univ. 28 (2021) 370–385.
K. Taguchi, H. Ono-nakazato, T. Usui, K. Marukawa, K. Katogi, H. Kosaka, ISIJ Int. 45 (2005) 1572–1576.
K. Suzuki, S. Ban-ya, M. Hino, ISIJ Int. 41 (2001) 813–817.
K. Suzuki, S. Ban-ya, M. Hino, ISIJ Int. 42 (2002) 146–149.
M. Paek, J. Jang, J. Jo, L. Holappa, J. Pak, Metall. Mater. Trans. B 52 (2020) 236–244.
X. Zhang, B. Xie, H.Y. Li, J. Diao, C.Q. Ji, Ironmak. Steelmak. 40 (2013) 282–289.
J.F. Xu, F.X. Huang, X.H. Wang, C.L. Jing, Steel Res. Int. 87 (2016) 1694–1701.
D.G.C. Robertson, B. Deo, S. Ohguchi, Ironmak. Steelmak. 11 (1984) 41–55.
S. Ohguchi, D.G.C. Robertson, B. Deo, P. Grieveson, J.H.E. Jeffes, Ironmak. Steelmak. 11 (1984) 202–213.
L. Jonsson, D. Sichen, P. Jonsson, ISIJ Int. 38 (1998) 260–270.
R.J. Pomfret, P. Grieveson, Can. Metall. Quart. 22 (1983) 287–299.
A.N. Conejo, F.R. Lara, M. Macias Hernández, R.D. Morales, Steel Res. Int. 78 (2007) 141–150.
G. Okuyama, K. Yamaguchi, S. Takeuchi, K. Sorimachi, ISIJ Int. 40 (2000) 121–128.
S. Kitamura, T. Kitamura, E. Aida, R. Sakomura, T. Kanek, T. Nuibe, ISIJ Int. 31 (1991) 1329–1335.
S.C. Duan, X. Shi, M.C. Zhang, B. Li, W.S. Yang, F. Wang, H.J. Guo, J. Guo, Metall. Mater. Trans. B 51 (2019) 353–364.
K. Ashok, G.K. Mandal, D. Bandyopadhyay, Trans. Indian Inst. Met. 68 (2015) 9–18.
S.J. Li, G.G. Cheng, Y. Huang, W.X. Dai, Z.Q. Miao, J. Iron Steel Res. Int. 27 (2020) 380–391.
S.J. Li, G.G. Cheng, L. Yang, L. Chen, Q.Z. Yan, C.W. Li, ISIJ Int. 57 (2017) 713–722.
X.M. Yang, C.B. Shi, M. Zhang, G.M. Chai, F. Wang, Metall. Mater. Trans. B 42 (2011) 1150–1180.
X.M. Yang, M. Zhang, C.B. Shi, G.M. Chai, J. Zhang, Metall. Mater. Trans. B 43 (2012) 241–266.
X.M. Yang, J.P. Duan, C.B. Shi, M. Zhang, Y.L. Zhang, J.C. Wang, Metall. Mater. Trans. B 42 (2011) 738–770.
S.C. Duan, C. Li, X.L. Guo, H.J. Guo, J. Guo, W.S. Yang, Ironmak. Steelmak. 45 (2018) 655–664.
S.M. Jung, ISIJ Int. 43 (2003) 216–223.
T. Itoh, T. Nagasaka, M. Hino, ISIJ Int. 40 (2000) 1051–1058.
L.C. Zhang, Y.P. Bao, M. Wang, C.J. Zhang, Int. J. Miner. Metall. Mater. 23 (2016) 408–416.
S.C. Duan, X.L. Guo, H.J. Guo, J. Guo, Ironmak. Steelmak. 44 (2017) 168–184.
J.X. Chen, Handbook of common figures, tables and data for steelmaking, 2nd Ed., Metallurgical Industry Press, Beijing, China, 2010.
X.M. Yang, J.S. Jiao, R.C. Ding, C.B. Shi, H.J. Guo, ISIJ Int. 49 (2009) 1828–1837.
J. Guo, S.W. Han, X.R. Chen, H.J. Guo, Y. Yan, Metall. Mater. Trans. B 51 (2020) 1813–1823.
Y. Ren, L.F. Zhang, W. Fang, S.J. Shao, J. Yang, W.D. Mao, Metall. Mater. Trans. B 47 (2015) 1024–1034.
X.R. Chen, G.G. Cheng, Y. Li, Y.Y. Hou, Metall. Res. Technol. 116 (2019) 626.
G.J. Chen, J. Yang, L. Li, M. Zhang, S.P. He, J. CO2 Util. 50 (2021) 101586.
Acknowledgements
This work was financially supported by Key R&D Plan of Shandong Province in 2021 (Grant No. 2021CXGC010209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, Y., Shang, Gh., Zhang, Lp. et al. A deoxidation thermodynamic model for 304 stainless steel considering multiple-components coupled reactions. J. Iron Steel Res. Int. 31, 74–91 (2024). https://doi.org/10.1007/s42243-023-01054-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-023-01054-9