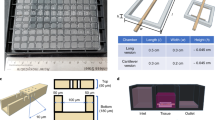
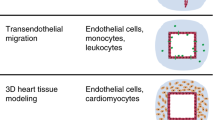
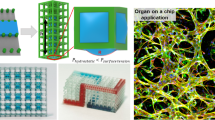
Abstract
A combination of hydrogels and microfluidics allows the construction of biomimetic three-dimensional (3D) tissue models in vitro, which are also known as organ-on-a-chip models. The hydrogel patterning with a well-controlled spatial distribution is typically achieved by embedding sophisticated microstructures to act as a boundary. However, these physical barriers inevitably expose cells/tissues to a less physiologically relevant microenvironment than in vivo conditions. Herein, we present a novel dissolvable temporary barrier (DTB) strategy that allows robust and flexible hydrogel patterning with great freedom of design and desirable flow stimuli for cellular hydrogels. The key aspect of this approach is the patterning of a water-soluble rigid barrier as a guiding path for the hydrogel using stencil printing technology, followed by a barrier-free medium perfusion after the dissolution of the DTB. Single and multiple tissue compartments with different geometries can be established using either straight or curved DTB structures. The effectiveness of this strategy is further validated by generating a 3D vascular network through vasculogenesis and angiogenesis using a vascularized microtumor model. As a new proof-of-concept in vasculature-on-a-chip, DTB enables seamless contact between the hydrogel and the culture medium in closed microdevices, which is an improved protocol for the fabrication of multiorgan chips. Therefore, we expect it to serve as a promising paradigm for organ-on-a-chip devices for the development of tumor vascularization and drug evaluation in the future preclinical studies.
Graphic abstract












Similar content being viewed by others
References
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32(8):760–772. https://doi.org/10.1038/nbt.2989
Chan CY, Huang PH, Guo F et al (2013) Accelerating drug discovery via organs-on-chips. Lab Chip 13(24):4697–4710. https://doi.org/10.1039/c3lc90115g
Low LA, Tagle DA (2017) Tissue chips–innovative tools for drug development and disease modeling. Lab Chip 17(18):326–336. https://doi.org/10.1039/c7lc00462a
Van De Stolpe A, Den Toonder J (2013) Workshop meeting report organs-on-chips: human disease models. Lab Chip 13(18):3449–3470. https://doi.org/10.1039/c3lc50248a
Wang X, Sun Q, Pei J (2018) Microfluidic-based 3D engineered microvascular networks and their applications in vascularized microtumor models. Micromachines 9(10):493. https://doi.org/10.3390/mi9100493
Kim S, Lee H, Chung M et al (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13(8):1489–1500. https://doi.org/10.1039/c3lc41320a
Zheng Y, Chen JM, Craven M et al (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109(24):9342–9347. https://doi.org/10.1073/pnas.1201240109
Li QY, Niu K, Wang D et al (2022) Low-cost rapid prototyping and assembly of an open microfluidic device for a 3D vascularized organ-on-a-chip. Lab Chip 22(14):2682–2694. https://doi.org/10.1039/d1lc00767j
Hsu YH, Moya ML, Abiri P et al (2013) Full range physiological mass transport control in 3D tissue cultures. Lab Chip 13(1):81–89. https://doi.org/10.1039/c2lc40787f
Chen MB, Whisler JA, Jeon JS et al (2013) Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol 5(10):1262–1271. https://doi.org/10.1039/c3ib40149a
Yeon JH, Ryu HR, Chung M et al (2012) In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 12(16):2815–2822. https://doi.org/10.1039/c2lc40131b
Vulto P, Podszun S, Meyer P et al (2011) Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab Chip 11(9):1596–1602. https://doi.org/10.1039/c0lc00643b
Trietsch SJ, Israëls GD, Joore J et al (2013) Microfluidic titer plate for stratified 3D cell culture. Lab Chip 13(18):3548–3554. https://doi.org/10.1039/c3lc50210d
Wang XL, Phan DTT, Sobrino A et al (2016) Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16(2):282–290. https://doi.org/10.1039/c5lc01050k
Cho H, Kim HY, Kang JY et al (2007) How the capillary burst microvalve works. J Colloid Interface Sci 306(2):379–385. https://doi.org/10.1016/j.jcis.2006.10.077
Wong AP, Perez-Castillejos R, Christopher Love J et al (2008) Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials 29(12):1853–1861. https://doi.org/10.1016/j.biomaterials.2007.12.044
Loessberg-Zahl J, Beumer J, van den Berg A et al (2020) Patterning biological gels for 3D cell culture inside microfluidic devices by local surface modification through laminar flow patterning. Micromachines 11(12):1112. https://doi.org/10.3390/mi11121112
Yamada A, Renault R, Chikina A et al (2016) Transient microfluidic compartmentalization using actionable microfilaments for biochemical assays, cell culture and organs-on-chip. Lab Chip 16(24):4691–4701. https://doi.org/10.1039/c6lc01143h
Tibbe MP, Leferink AM, van den Berg A et al (2018) Microfluidic gel patterning method by use of a temporary membrane for organ-on-chip applications. Adv Mater Technol 3(3):1700200. https://doi.org/10.1002/admt.201700200
Pei JH, Sun QY, Yi ZR et al (2020) Recoverable elastic barrier for robust hydrogel patterning with uniform flow profile for organ-on-a-chip applications. J Micromech Microeng 30(3):35005. https://doi.org/10.1088/1361-6439/ab68b2
Goldschmidtboeing F, Rabold M, Woias P (2006) Strategies for void-free liquid filling of micro cavities. J Micromech Microeng 16(7):1321–1330. https://doi.org/10.1088/0960-1317/16/7/029
Chibbaro S, Costa E, Dimitrov DI et al (2009) Capillary filling in microchannels with wall corrugations: a comparative study of the Concus−Finn criterion by continuum, kinetic, and atomistic approaches. Langmuir 25(21):12653–12660. https://doi.org/10.1021/la901993r
Wang XL, Phan DTT, Zhao D et al (2016) An on-chip microfluidic pressure regulator that facilitates reproducible loading of cells and hydrogels into microphysiological system platforms. Lab Chip 16(5):868–876. https://doi.org/10.1039/c5lc01563d
Sobrino A, Phan DTT, Datta R et al (2016) 3D microtumors in vitro supported by perfused vascular networks. Sci Rep 6(1):31589. https://doi.org/10.1038/srep31589
Phan DTT, Wang XL, Craver BM et al (2017) A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 17(3):511–520. https://doi.org/10.1039/c6lc01422d
Li S, Butler P, Wang YX et al (2002) The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA 99(6):3546–3551. https://doi.org/10.1073/pnas.052018099
Laco F, Grant MH, Black RA (2013) Collagen-nanofiber hydrogel composites promote contact guidance of human lymphatic microvascular endothelial cells and directed capillary tube formation. J Biomed Mater Res A 101A(6):1787–1799. https://doi.org/10.1002/jbm.a.34468
Ivanov KP, Kalinina MK, Levkovich YI (1981) Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc Res 22(2):143–155. https://doi.org/10.1016/0026-2862(81)90084-4
Patan S (2004) Vasculogenesis and angiogenesis. Cancer Treat Res 117:3–32. https://doi.org/10.1007/978-1-4419-8871-3_1
Kim SD, Chung M, Ahn J et al (2016) Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip 16(21):4189–4199. https://doi.org/10.1039/c6lc00910g
Nashimoto Y, Hayashi T, Kunita I et al (2017) Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol 9(6):506–518. https://doi.org/10.1039/c7ib00024c
Bockhorn M, Jain RK, Munn LL (2007) Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 8(5):444–448. https://doi.org/10.1016/S1470-2045(07)70140-7
Primo GA, Mata A (2021) 3D patterning within hydrogels for the recreation of functional biological environments. Adv Funct Mater 31(16):2009574. https://doi.org/10.1002/adfm.202009574
Miri AK, Mirzaee I, Hassan S et al (2019) Effective bioprinting resolution in tissue model fabrication. Lab Chip 19(11):219–237. https://doi.org/10.1039/c8lc01037d
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31972929 and 62231025), the Research Program of Shanghai Science and Technology Committee (Nos. 21140901300 and 20DZ2220400), the Natural Science Foundation of Chongqing, China (No. CSTB2022NSCQ-MSX0767), the Interdisciplinary Program of Shanghai Jiao Tong University (Nos. YG2021ZD22 and YG2023LC04), the Foundation of National Center for Translational Medicine (Shanghai) SHU Branch (No. SUITM-2023008), and the Cross-disciplinary Research Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYJC202108). The authors were also grateful to the Center for Advanced Electronic Materials and Devices (AEMD) of Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Contributions
DW and QYL were involved in conceptualization, investigation, methodology, writing—original draft, and visualization; CYZ, ZJL, KYL, YJL, and LX helped in resources and writing—review and editing; XLW contributed to supervision and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Ding Wang and Qinyu Li have contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Li, Q., Zhou, C. et al. Dissolvable temporary barrier: a novel paradigm for flexible hydrogel patterning in organ-on-a-chip models. Bio-des. Manuf. 7, 153–166 (2024). https://doi.org/10.1007/s42242-023-00267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-023-00267-x