Abstract
Antagonism against Botrytis cinerea is often carried out using yeast as direct antagonists. Aureobasidium pullulans strain AP1 was tested in two different formulations: wettable powder (WP) and oil dispersion (OD). By in vitro assays, the viability of the strain cells was constantly evaluated for seven months and the OD formulation ensured the highest cells viability. The efficacy of the formulations was assayed by evaluating the production of volatile and non-volatile metabolites. Results showed that the formulation affected the non-volatile less than the volatile metabolites. Both AP1 WP and AP1 OD non-volatile metabolites displayed almost 50% of mycelial pathogen inhibition. Comparing the two products, the lowest EC50 value (518.15 mg L− 1) was detected for the AP1 OD formulation that was thus chosen for postharvest in vivo assays. The preventative treatments (200, 400, 800 mg L− 1) were active in reducing the pathogen incidence on table grape on average by 52%. Instead, in the curative application assay, the highest concentration (800 mg L− 1) reduced grey mold incidence by 86%. The present study reported the potential of two new formulations to use against the postharvest grey mold of table grape for a possible further commercial product development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Table grape is an important fruit crop, with over 31 million tons produced worldwide (FAO, 2021). Sensorial attributes jointly with health-promoting compounds, make this fruit highly appreciated by consumers (De Simone et al. 2020; Pezzuto 2008). On another hand, Botrytis cinerea is the most important pathogen of grape berries, as causal agent of grey mould disease. The fungal pathogen causes economically relevant losses both before and after harvest (Xu et al. 2007). Synthetic fungicides are generally used to control this pathogen, but in the last years, their use in agriculture has been associated with growing environmental and public health concerns (Lázaro et al. 2021). European Union (EU) through the ‘Farm-to-Fork’ strategy is also boosting the increase of the use of agricultural land under organic farming, leading to the search of alternatives to synthetic molecules, such as the use of microbial formulates (Casals et al. 2021).
Yeasts are among the most studied biocontrol agents (BCAs) for the multiple mechanisms of action that they are able to enact (Agirman and Erten 2020; Di Francesco et al. 2018; Droby et al. 2016; Freimoser et al. 2019), and Aureobasidium pullulans is a prominent option as antagonist of B. cinerea. In fact, several studies delineated the efficacy of A. pullulans against this pathogen (De Simone et al. 2020; Fedele et al. 2020; Di Francesco et al. 2018). In this context, it is worth mentioning that several criteria must be considered when a formulated product is developed, such as the selected strains’ antagonistic efficacy, their performance in the field and environmental impact, and the costs and marketing aspects (Teixidó et al. 2022). For this reason, the formulation is the most important and challenging step in the development and commercialization of a bio-based product (Carbó et al. 2019). The production starts with the fermentation of the active ingredient (the selected BCA or BCAs) in an optimized medium and growth conditions (Droby et al. 2016); once the necessary amount of the biomass has been obtained, the downstream process starts with the stabilization of the cells achieved through refrigeration, freeze-drying, and dehydration (Droby et al. 2016; Teixidó et al. 2022). The stabilized active ingredient can be commercialized in different forms, such as dried powder or granules, liquid, oil, or water based (Droby et al. 2016; Teixidó et al. 2022).
It is important to stress that the co-formulants can modify the efficacy and the performance of a product, enhancing the potential of the microorganisms or adding side effects on the target pathogen (Bühlmann et al. 2021; Carbó et al. 2019; Lopes et al. 2011; Wraight and Ramos 2017).
Based on these facts, the objectives of the present study were (i) to evaluate the antifungal activity against B. cinerea on table grape of two different formulations, a wettable powder (WP) and an oil dispersion (OD), both based on an A. pullulans strain from the mycological collection of Di4A- Department of Agriculture, Food, Environmental and Animal Sciences of Udine University, by in vitro (ii) and in vivo assays; (iii) to assess the viability over time of the strain used in both formulations.
Material and methods
Biocontrol agent and formulated products
Aureobasidium pullulans strain AP1, belonging to the mycological collection of Di4A-University of Udine, was selected among different strains on the bases of previously tested antagonistic capabilities and formulation viability (data not shown). The strain was used as active ingredient for two formulation prototypes, developed in collaboration with Clever Bioscience s.r.l. (Campospinoso, Italy). The yeast was formulated as a wettable powder (WP) mainly composed of inert compounds, and as oil dispersion (OD), prepared with food grade and eco-friendly surfactants (provided by Clever Bioscience, composition not disclosable). The biomass of the microorganism was obtained after 24 h of fermentation at 25 °C on NYDB medium (8 g Nutrient broth, 5 g Yeast extract, 10 g Dextrose in 1 L of distilled water) (Oxoid, UK) in a MiniIO reactor (Solaris, Italy) where sterile air insufflation and the pH parameters were set to 100% (O2) and to 6.5, respectively. After 24 h, the liquid substrate was centrifuged, and the supernatant discarded. The yeast cells were washed with physiological solution (9 g L− 1 of NaCl) before being mixed with the cryoprotectant (provided by Clever bioscience srl, composition not disclosed) and freeze-dried (FreeZone® 4.5 L Benchtop Freeze Dryers, VWR, USA). The concentration of the viable active ingredient was set to 1 × 108 cells g− 1 of both products, WP and OD. As positive controls, the pure strain culture AP1 (1 × 107 cells mL − 1) and the commercial product Botector® New (A. pullulans strains DSM 14,940 and DSM 14,941) (Manica, Italy) purchased for the experiments (400 mg L − 1, as suggested by the technical data sheet), were used as references. The yeast was cultured on NYDA medium (as above and with 25 g Technical Agar) for 2 days at 25 °C. AP1 cells were collected and suspended in sterile distilled water (SDW) containing 0.05% (v/v) Tween 20 and the suspension concentration was adjusted by using a hemacytometer.
Pathogen and fruit
Botrytis cinerea strain Bc1 used in this work belonged to the mycological collection of Di4A-Department of Agriculture, Food, Environmental and Animal Sciences of Udine University and it was originally isolated from grapefruit cv “Sugraone”. For the in vitro and in vivo experiments, 7-days-old colonies cultured on PDA medium (39 g of Potato Dextrose Agar in 1 L of distilled water) (Oxoid, UK) at 20 °C were used. Regarding the in vivo assays, table grape cv “Black Magic” was bought in the local market at the right maturity stage (18 °brix) and without any visible diseases or defects.
Formulations’ shelf-life
The viability of both formulation prototypes was evaluated 4 times in 7 months, as described by Bühlmann et al. (2021). One gram of each product was diluted in 10 mL of SDW. The suspension was shaken for 5 min in an orbital shaker, and 10-fold dilutions were subsequently made by using SDW. Then, 100 µL of each dilution were plated on NYDA plates and incubated at 25 °C for 2 days. The sample unit for each dilution and formulation consisted in 5 plates.
In vitro assay: evaluation of formulated products efficacy against B. cinerea
The mechanism of action of antibiosis was investigated focusing the study on the production of volatile and non-volatile metabolites so evaluating the effect of the two formulations (Di Francesco et al. 2020). The efficacy of the non-volatile compounds was tested by using a modified version of the protocol described by Di Francesco et al. (2023). Both formulation prototypes, strain AP1, and Botector® New, were inoculated in liquid media, NYDB and Grape Juice Broth (GJB) (250 mL), contained in sterile flasks, by maintaining the final concentration of 1 × 107 cells mL− 1, in constant agitation (150 rpm) at 20 °C. After two days, the liquid cultures were centrifuged, and the supernatants were filtered by 0.22 μm sterile syringe filters (Sarstedt, Germany). NYDB and GJB filtrates (200 mL) were added each to 200 mL of SDW having 6 g of Technical Agar (Oxoid, UK). Each medium was plated and inoculated with a mycelial plug (6 mm diameter) of a 7-days-old colony of B. cinerea (Bc1). Plates were incubated at 20 °C and the pathogen colony diameter was measured after 3 days by using a caliber.
For the evaluation of the effectiveness of VOCs produced by the target treatments, a double Petri dish assay was performed according to the method of Roussi et al. (2013). The two formulates, AP1 strain, and Botector® New suspensions (100 µL) were spread on NYDA and GJA (15 g of Technical Agar -Oxoid, UK- in 250 mL of organic red grape juice, and 750 mL of distilled water) media, maintaining the above-cited concentrations. The plates were incubated at 25 °C for two days. Respectively, PDA and GJA plates were inoculated with a mycelial plug of the isolate Bc1 (6 mm diameter) and immediately joined to the previously prepared plates by using a double layer of Parafilm® (Amcor, USA), maintaining the match NYDA/PDA and GJA/GJA. Plates were incubated at 20 °C. After 3 days, the pathogen colony diameter was measured. The sample unit was 5 plates for each condition. The control was constituted by NYDA and GJA plates not inoculated for the first assay and by NYDA and GJA plates inoculated with 100 µL of SDW for the second assay. The experiments were conducted twice.
Formulations’ EC50 values
To define the EC50 value of both formulations, five different concentrations of each were used to create a baseline. Hence 600 mg L-1, 400 mg L-1, 200 mg L-1, 100 mg L-1, and 50 mg L-1 of each product were used to amend PDA medium (Oxoid, UK). A 6 mm diameter fungal mycelial plug was placed in the centre of the petri dishes. The plates were incubated at 20 °C and after 3 days the colony diameter was recorded. The sample unit was composed of 5 plates for each concentration and formulation, while the control was PDA with no amendment. The assay was conducted twice. The pathogen inhibition percentages were used to determine the EC50 values of both formulations. To calculate the inhibition values, the following equation was used (Chen and Dai, 2012):
Where (%) is the percentage of inhibition of pathogen mycelial growth, while d1and d2 are the control colony diameter (mm) and the treated colony diameter (mm), respectively.
Preventative and curative efficacy of yeast formulations at different concentrations against B. cinereaon table grape
In in vivo assays, the OD bioproduct, which displayed the best efficacy against the pathogen by in vitro assays, was applied as preventative and curative treatment at different concentrations, based on the previously determined EC50 values. Thirty berries of table grape cv “Black magic” per 3 independent replicates were used. For each experiment fruits were washed very carefully in a sodium hypochlorite solution (1%) and then rinsed with tap water. Once dried, table grapes were punched with a sterile needle and then inoculated. For the curative assay, grapes were inoculated with 15 µL of B. cinerea (1 × 105 conidia mL − 1) and for the preventative assay, with 15 µL of three different bioproduct concentrations (800 mg L − 1, 400 mg L − 1, and 200 mg L − 1) defined previously on the bases of the EC50 values, i.e. the concentrations of the compound that gives half-maximal response. After 2 h, fruits were inoculated with the same volume and in the same wound with the different bioproduct concentrations and Bc1 suspension, respectively for the preventative and the curative assay. Grapes were stored in sterile plastic boxes (18 cm×28 cm× 9 cm, L×W×H) at 20 °C, and the incidence and the severity of the disease were evaluated after 3 days for both assays. The assay was conducted twice. As negative control, grapes were inoculated instead of treatments with SDW. As positive control, Botector® New (0.4 g mL − 1) was used.
Data analysis
Data were analysed by ANOVA one-way analysis. The separation of means was performed with a Tukey’s test (α = 0.05, α = 0.01) by using the software MiniTab.16. Data were reported as mean values ± standard error (SE). The EC50 of each bioproduct, i.e. the concentration of the compound that gives half-maximal response, was calculated using the probit analysis applied to the percentage of mycelial colony growth (Lesaffre and Molenberghs 1991).
Results
Formulations’ viability
The viability of the two formulation prototypes was conducted over time to verify the shelf life of the products during the storage at 4 °C. Figure 1 displayed a similar trend between the two formulates. Regarding AP1 OD formulation, the cells viability reduction was substantially constant during the 7 months of storage (from February 2023 to September 2023), except for a significant cells’ reduction viability, from 1 × 108 CFU g− 1 to 5 × 106 CFU g− 1, that was detected after 4 months (T2, June) of storage at 4 °C. However, the bioformulation resulted stable for the subsequent 3 months (T3, September).
Aureobasidium pullulans AP1 formulated products cell viability. Each point represents the mean of the number of colonies forming units (Log10 CFUs) from 5 replicates for each sampling time (T0 = February 2023, T1 = April 2023, T2 = June 2023, T3 = September 2023). The products were stored at 4 °C. Different letters indicate significant differences according to Tukey’s test (α = 0.05)
With reference to AP1 WP formulation, after 4 and 7 months of storage, cells viability significantly decreased from the starting value’s 1 × 108 CFU g− 1 to 7.9 × 106 CFU g− 1 and 6.6 × 106 CFU g− 1, respectively.
In vitro assays
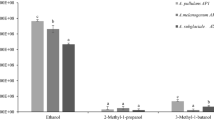
The formulations were tested to assess the effectiveness of the yeast strain following the formulation process, which could alter the performance of the active ingredient (AP1). Thus, the activity of non-volatile and volatile metabolites on B. cinerea growth was investigated. Figure 2A reports the efficacy of the treatments in the No-VOCs assays against the fungal pathogen. The most effective formulation in reducing the pathogen growth was AP1 OD, which was able to reduce by 47.5% and by 64.0% the pathogen in the PDA and GJA amended media, respectively. The AP1 WP formulation was effective as the above-mentioned AP1 OD only on PDA amended medium. AP1 strain metabolites were only effective on PDA medium (23.5% of reduction) against B. cinerea, while on GJA no inhibition was detected. The positive control did not restrict Bc1 growth, but conversely stimulated its colony growth (+ 14%) on GJA.
Effect of non-volatile (A) and volatile (B) metabolites produced by the two formulations (AP1 WP and AP1 OD), the strain AP1 and Botector® New, on the mycelial growth (mm) of Botrytis cinerea (Bc1). For non-volatile assay, the fungus was cultured on agar amended with treatments culture filtrates previously grown for 2 days in NYDB and Grape Juice Broth (GJB), respectively. For VOCs, treatments (100 µL) were spread on NYDA and GJA media and joined respectively with PDA and GJA plates inoculated with the pathogen. Fungal colony diameter (mm) was measured after 3 days of incubation at 20 °C. Each value is the mean of 5 plates (replicates) ± standard error. Different letters indicate significant differences according to Tukey’s Test (α = 0.01) within the same medium
VOCs efficacy was less noticeable with respect to that of No-VOCs metabolites (Fig. 2B). However, the two formulations acted better than the other solutions against grey mould. On PDA-amended agar, the AP1 WP inhibited by 39% the growth of B. cinerea with respect to the control. In the case of GJA, the AP1 OD bioproduct reduced the growth of Bc1 by 21.2%, compared to the control. The two bioformulations outperformed the pure AP1 strain in producing active secondary metabolites (Fig. 3). The positive control slightly reduced the growth on the PDA medium by producing volatile compounds, but no effect was noticed in the No-VOCs assay.
EC50 values
The two formulations, AP1 OD and AP1 WP, were tested for their inhibitory activity on the mycelial growth of B. cinerea Bc1 by testing five different concentrations. EC50 values were calculated on the basis of Bc1 colony inhibition rate determined by the different concentrations of both formulates. Comparing the two products, the lowest EC50 value (518.15 mg L− 1) was detected for the AP1 OD bioformulation. Conversely, much higher EC50 value (1078.9 mg L− 1) was found for the AP1 WP formulation.
OD formulate in vivo efficacy on table grape
Formulate AP1 OD was chosen for the in vivo application on the basis of previous in vitro assays results and EC50 detected values. The formulate resulted effective in controlling the grey mould incidence on table grapes by reducing the number of infected berries in treated samples to 40% (on average between the tested concentrations) and 10% (by using the concentration 800 mg L − 1) for preventive and curative applications, respectively, with respect to the controls (95% and 75% of rotten berries). In the case of preventative application (Fig. 4A), the three tested different concentrations were not statistically different among them. Conversely, the 800 mg L− 1 curative application (Fig. 4B) was more effective compared to the 200 mg L− 1, greatly reducing the pathogen growth, and allowing only 10% of rotten fruits. On another hand, the severity of the lesions in the diseased fruits was less affected by the treatment. In the case of preventative applications (Fig. 5A), both the commercial product (Botector® New) and AP1OD formulation reduced by 22% the disease lesion diameter, without statistical significance among the treatments (Fig. 6). No relevant differences were noticed for the lesion diameter values (Fig. 5B) in the case of the curative application of the treatments, the only note was that AP1 OD at 200 mg L− 1 slightly stimulated pathogen aggressiveness.
Efficacy of different concentrations (200, 400, 800 mg L− 1) of AP1 OD formulation on Botrytis cinerea disease incidence (%) on table grape after a preventative (A) and a curative (B) application on artificially wounded berries. Each value is the mean of the disease incidence detected in 3 replicates ± standard error. Different letters indicate significant differences according to Tukey’s Test (α = 0.05)
Efficacy of different concentrations (200, 400, 800 mg L− 1) of AP1 OD formulation on Botrytis cinerea disease severity (mm) on table grape after preventative (A) and curative (B) application on artificially wounded berries. Each value is the mean of 30 berries ± standard error. Different letters indicate significant differences according to Tukey’s Test (α = 0.05)
Three different AP1OD bioproduct concentrations (800 mg L -1, 400 mg L -1, and 200 mg L -1) were used as preventative treatments against Botrytis cinerea (1×105 conidia mL -1) on table grape cv “Black Magic”. Negative and positive controls were represented by sterile water and Botector® New (0,4 g mL -1), respectively.
Discussion
Aureobasidium pullulans is a well-known BCA active against many postharvest fungal pathogens in various fruits such as citrus, peach, apple (Parafati et al. 2016; Klein and Kupper 2018) and in particular against B. cinerea on table grape, kiwifruit, and strawberries (Di Francesco et al. 2020; Iqbal et al. 2023). Although a large number of studies focused on its modes of action, only rarely its antagonistic activity as active ingredient of a formulation has been reported. This is a key point to better understand the application and effectiveness of these products in planning the application modes to suit particular postharvest conditions (Chanchaichaovivat et al. 2008). Several studies reported the antagonistic activity of BCAs with the aim of understanding their modes of action, and hence facilitate their formulation and subsequently their registration for a commercial use. Current trends toward the reduction of the use of synthetic fungicides are supported by a strong public and scientific desire to seek safer and eco-friendly alternatives to reduce postharvest losses (Mari et al. 2014; Di Francesco et al. 2023). However, there is a limited use of these formulations due to the ineffectiveness and control variability of BCAs under commercial conditions (Droby et al. 2009). The reduced efficacy with respect to fresh cells, high production costs, and the registration barriers justify the limited impact on the market of the formulations (Yu et al. 2012). Since a shelf-life of at least 6 months is a major goal for a formulation we measured the viability of the yeast cells in our OD and AP formulations over a period of seven months (Yu et al. 2012; Mari et al. 2014).
Our results displayed how both the tested formulations, during seven months of storage, maintained the viability of the yeast cells. The AP1 OD formulation ensured a greater cells viability overtime if compared to the AP1 WP formulation, probably due to the oil protective action against oxidative stresses and the regulation of water exchanges (Lopes et al. 2011; Mbarga et al. 2014).
In fact, both prototypes resulted more stable compared to a highly concentrated suspension of fresh cells in water and glycerol, which lost 99% of viability in 2 weeks (data not shown). As it has been reported that formulations could influence the behaviour of the active ingredient (Wraight and Ramos 2017), the effectiveness of secondary metabolites produced by AP1 strain and by the formulated prototypes, were confirmed through in vitro assays. Moreover, an interesting result emerges from the fact that both prototypes appear to enhance the antagonistic activity of the strain, thus bypassing the issue related to bio-formulates concerning the limited performance of the active strain under commercial conditions (Droby et al. 2009). The results, on common media (e.g. NYDA, PDA), highlighted the influence of the co-formulants on the inhibition efficacy exerted by AP1, remarking differences in the behaviour of the BCA and the efficacy of its metabolites.
Regarding the assay with GJA medium, only the AP1 OD formulation was able to reduce B. cinerea up to 64% by non-VOCs metabolites. Instead, the inhibition rate by AP1 OD formulate volatile compounds on GJA, a rich medium, was lower with respect to non-VOCs, displaying 23% of mycelial colony inhibition.
These results highlighted the importance of the medium where the microorganisms grow (Hamdache et al. 2019), confirming that BCAs inhibitory effect was reduced by high nutrients concentrations that instead stimulated pathogen growth (Liu et al. 2013).
The use of GJA on in vitro assay aimed to simulate the real application environment of the bioformulation prototypes. Based on the EC50 results, obtained from baseline concentrations of both formulations against B. cinerea mycelial growth, the OD formulation was selected for in vivo assay on table grape to counter grey mould incidence and severity, in preventative and curative treatments. We noticed that the effectiveness of the different concentrations used, ranging from 200 to 800 mg/L, was linked to application times. Concerning the preventative effect, the three different concentrations of AP1 OD behaved similarly on grape berries, reducing the pathogen incidence by 52% with respect to the controls. This result may help defining the right application dosage of a possible commercial formulation.
In the curative application assay, grey mould incidence was reduced by 86% with respect to the untreated control by the highest tested concentration (800 mg L− 1). Nevertheless, both concentrations 200 and 400 mg L− 1, notably reduced the pathogen incidence (63% on average), showing a higher fungal inhibition rate than Botector® New (26%), as compared to the untreated control (water). Another interesting result emerged from the grey mould severity lesions on fruits that were slightly stimulated by the treatments as curative, allowing to speculate that B. cinerea, after succeeding in colonizing the wound, may take advantage of the co-formulants.
The technological properties of the formulation are clues for the explanation of the different behaviour as discussed above. Oil Dispersion (OD) guarantees an increased persistence and dispersion of BCAs cells on the surface of the host (Mbarga et al. 2014). As far as we know, this study is a first report on the efficacy of a formulated yeast strain in an OD formula for use against postharvest pathogenic fungi. The effectiveness of oil coating containing BCAs has been the subject of several studies, showing that this kind of formulation creates a modified atmosphere around the treated fruit, sustaining the antagonist growth (Yu et al. 2012; Mari et al. 2014). Mbarga et al. (2014) successfully developed an OD product to control the black pod disease of cocoa.
Conclusion
The present study assessed the formulation technological characteristics, the in vivo efficacy, and the long-term stability, giving hope for a possible further commercial product development. In fact, the main factors limiting commercial interest in biocontrol-oriented products are usually the high costs of production, the culture substrates, and the low biomass productivity together with the entire bioformulation regulatory process (Mari et al. 2014). However, in view of a commercial formulation, more studies are necessary such as the genomic characterization of the active strain, the detection of the persistence of the BCA on the fruit surface and the possible effects of the formulation on the fruit’s sensory properties and residual issues.
Data availability
Data supporting this research is available upon reasonable request.
References
Agirman B, Erten H (2020) Biocontrol ability and action mechanisms of Aureobasidium pullulans GE17 and meyerozyma guilliermondii KL3 against Penicillium Digitatum DSM2750 and Penicillium Expansum DSM62841 causing postharvest diseases. Yeast 37:437–448. https://doi.org/10.1002/yea.350
Bühlmann A, Kammerecker S, Müller L, Hilber-Bodmer M, Perren S, Freimoser FM (2021) Stability of dry and liquid Metschnikowia pulcherrima formulations for biocontrol applications against apple postharvest diseases. Horticolturae 7:459. https://doi.org/10.3390/horticulturae7110459
Carbó A, Teixidó N, Usall J, Torres R (2019) Verifying the biocontrol activity of novel film-forming formulations of Candida sake CPA‐1: resilience in relation to environmental factors, rainfall episodes, and control of Botrytis Cinerea on different hosts. J Sci Food Agric 99:4969–4976. https://doi.org/10.1002/jsfa.9731
Casals C, Guijarro B, De Cal A, Torres R, Usall J, Perdrix V, Hilscher U, Ladurner E, Smets T, Teixidó N (2021) Field validation of biocontrol strategies to control brown rot on stone fruit in several European countries. Pest Manag Sci 77:2502–2511. https://doi.org/10.1002/ps.6281
Chanchaichaovivat A, Ruenwongsa P, Panijpan B (2008) Screening, and identification of yeast strains from fruits and vegetables: potential for biological control of postharvest Chilli anthracnose (Colletotrichum Capsici). Biol Control 42:326–335. https://doi.org/10.1016/j.biocontrol.2007.05.016
Chen Y, Guanghui D (2012) Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber. J Sci Food Agric 92:1937–1943. https://doi.org/10.1002/jsfa.5565
De Simone N, Pace B, Grieco F, Chimienti M, Tyibilika V, Santoro V, Capozzi V, Colelli G, Spano G, Russo P (2020) Botrytis Cinerea and table grapes: a review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 9:1138. https://doi.org/10.3390/foods9091138
Di Francesco A, Mari M, Ugolini L, Baraldi E (2018) Effect of Aureobasidium pullulans strains against Botrytis cinerea on kiwifruit during storage and on fruit nutritional composition. Food Microbiol 72:67–72. https://doi.org/10.1016/j.fm.2017.11.010
Di Francesco A, Di Foggia M, Zajc J, Gunde-Cimerman N, Baraldi E (2020) Study of the efficacy of Aureobasidium strains belonging to three different species: A. Pullulans, A. subglaciale and A. melanogenum against Botrytis cinerea of tomato. Ann Appl Biol 177:266–275. https://doi.org/10.1111/aab.12627
Di Francesco A, Jabeen F, Di Foggia M, Zanon C, Cignola R, Sadallah A, Tugnoli V, Ermacora P, Martini M (2023) Study of the efficacy of bacterial antagonists against Cadophora luteo-olivacea of kiwifruit. Biol Control 180:105199. https://doi.org/10.1016/j.biocontrol.2023.105199
Droby S, Wisniewski M, Macarisin D, Wilson C (2009) Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol Technol 52:137–145. https://doi.org/10.1016/j.postharvbio.2008.11.009
Droby S, Wisniewski M, Teixidó N, Spadaro D, Jijakli MH (2016) The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol Technol 122:22–29. https://doi.org/10.1016/j.postharvbio.2016.04.006
European Commission (2020) Communication from the commission to the European Parliament, the council, the European economic and social committee and the committee of the regions. a farm to fork strategy for a fair, healthy and environmentally-friendly food system COM/2020/381 final
Fedele G, Brischetto C, Rossi V (2020) Biocontrol of Botrytis Cinerea on grape berries as influenced by temperature and humidity. Front Plant Sci 11:1232. https://doi.org/10.3389/fpls.2020.01232
Food and Agriculture Organization of the United Nations (2021) FAOSTAT Statistical Database. FAO, Rome
Freimoser FM, Rueda-Mejia MP, Tilocca B, Migheli Q (2019) Biocontrol yeasts: mechanisms and applications. World J Microbiol Biotechnol 35:154. https://doi.org/10.1007/s11274-019-2728-4
Hamdache A, Ezziyyani M, Lamarti A (2019) Study of growth and production of Botrytis Cinerea Conidia of some Morrocan isolates in different nutrients media. In: Ezziyyani M (ed) Advanced Intelligent systems for Sustainable Development (AI2SD’2018). Springer International Publishing, Cham, pp 62–68
Iqbal M, Broberg A, Andreasson E, Stenberg JA (2023) Biocontrol potential of beneficial fungus Aureobasidium Pullulans against Botrytis Cinerea and Colletotrichum Acutatum. Phytopathology 113:1428–1438. https://doi.org/10.1094/PHYTO-02-23-0067-R
Klein MN, Kupper KC (2018) Biofilm production by Aureobasidium Pullulans improves biocontrol against sour rot in citrus. Food Microbiol 69:1–10. https://doi.org/10.1016/j.fm.2017.07.008
Lázaro E, Makowski D, Vicent A (2021) Decision support systems halve fungicide use compared to calendar-based strategies without increasing disease risk. Commun Earth Envir 2:224. https://doi.org/10.1038/s43247-021-00291-8
Lesaffre E, Molenberghs G (1991) Multivariate probit analysis: a neglected procedure in medical statistics. Stat Med 10:1391–1403. https://doi.org/10.1002/sim.4780100907
Liu P, Li L, Chao-an L (2013) Characterization of competition for nutrients in the biocontrol of Penicillium Italicum by Kloeckera apiculata. Biol Control 67:157–162. https://doi.org/10.1016/j.biocontrol.2013.07.011
Lopes RB, Pauli G, Mascarin GM, Faria M (2011) Protection of entomopathogenic conidia against chemical fungicides afforded by an oil-based formulation. Biocontrol Sci Technol 21:125–137. https://doi.org/10.1080/09583157.2010.534548
Mari M, Di Francesco A, Bertolini P (2014) Control of fruit postharvest diseases: old issues and innovative approaches. Stew Postharvest Rev 10:1–4. https://doi.org/10.2212/spr.2014.1.1
Mbarga JB, Begoude BAD, Ambang Z, Meboma M, Kuate J, Schiffers B, Ewbank W, Dedieu L, Ten Hoopen GM (2014) A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora Megakarya. Biol Control 77:15–22. https://doi.org/10.1016/j.biocontrol.2014.06.004
Parafati L, Vitale A, Restuccia C, Cirvilleri G (2016) The effect of Locust bean gum (LBG)-based edible coatings carrying biocontrol yeasts against Penicillium Digitatum and Penicillium Italicum causal agents of postharvest decay of mandarin fruit. Food Microbiol 58:87–94. https://doi.org/10.1016/j.fm.2016.03.014
Pezzuto JM (2008) Grapes and human health: a perspective. J Agric Food Chem 56:6777–6784. https://doi.org/10.1021/jf800898p
Teixidó N, Usall J, Torres R (2022) Insight into a successful development of biocontrol agents: production, formulation, packaging, and shelf life as key aspects. Horticolturae 8:305. https://doi.org/10.3390/horticulturae8040305
Wraight SP, Ramos ME (2017) Effects of inoculation method on efficacy of wettable powder and oil dispersion formulations of Beauveria bassiana against Colorado potato beetle larvae under low-humidity conditions. Biocontrol Sci Technol 27:348–363. https://doi.org/10.1080/09583157.2017.1291904
Xu WT, Huang KL, Guo F, Qu W, Yang JJ, Liang ZH, Luo YB (2007) Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis Cinerea. Postharvest Biol Technol 46:86–94. https://doi.org/10.1016/j.postharvbio.2007.03.019
Yu S, Oh BT, Lee YH (2012) Biocontrol of green and blue molds in postharvest satsuma mandarin using Bacillus amyloliquefaciens JBC36. Biocontrol Sci Technol 22:1181–1197. https://doi.org/10.1016/j.micres.2022.127016
Acknowledgements
The PhD fellowship was co-financed by the European Project FSE REACT-EU, PON Research and Innovation 2014–2020 Axis IV Action IV.5.
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The research not involved human participants and/or animals.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cignola, R., Firrao, G., Freschi, G. et al. Aureobasidium pullulans formulations: evaluation of the effectiveness against grey mould of table grape. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01671-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01671-7