Abstract
Fire blight, caused by Erwinia amylovora, can be spread through orchards and nurseries because of poor sanitation practices such as contaminated secateurs. This research investigated the efficacy of 12 commercial sterilants at varying concentrations to kill E. amylovora on secateurs. Secateurs were dipped into an Erwinia amylovora inoculum solution (106 colony forming units/mL), and then misted with a test sterilant. After 10 s, secateurs were swabbed and plated onto Kings B agar plate, incubated at 26 °C for 48 h and bacterial colonies counted. Sterilants were also assessed for cotton bleaching and metal corrosion. Best performing sterilants were then tested as described above by cutting through infected plant material containing sticky bacterial ooze as inoculum. Each of the sterilants tested (methylated spirits (95% and 70%), sodium hypochlorite (NaOCl) (1%, 0.5%, 0.135%), Bac-Stop/benzalkonium chloride (2%, 1%), Virkon™ (label rate), Dettol (50%, 10%, 1%, 0.1%), and HarvestCide® gel (0.1%, 0.5%, 1%)) were found to be effective to kill E. amylovora on inoculum-coated secateurs. The best performing sterilants (methylated spirits, Dettol, HarvestCide gel, NaOCl) were also effective in killing E. amylovora on infected plant material when compared with the untreated control. Most sterilants, except methylated spirits or Dettol, caused corrosion of metal and bleaching of cotton. Each of the tested sterilants were found to be effective to kill Erwinia amylovora on inoculum-coated secateurs and inoculum in plant material and bacterial ooze. Many of the best performing sterilants were likely to damage tools over time and cause bleaching on clothing. However, Dettol or methylated spirits did not cause metal corrosion or bleaching.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire blight is caused by the bacterial pathogen Erwinia amylovora (Burrill 1882; Winslow et al. 1920). From as far back as the 1700s pear and apple growers have removed infected branches to control fire blight (Keil and van der Zwet 1967). Over the years, it has become a common recommendation to growers to remove infected material at an early infection stage to control the spread of the pathogen (Van der Zwet et al. 2012).
General overall sanitation practices remain an important first step in reducing or managing fire blight. This includes the removal of blighted shoots during the growing season or removal of overwintering cankers. Research over a long period of time has shown that the decontamination of pruning tools helps mitigate against the transmission of the bacteria when pruning out fire blight. In 1898, Waite was one of the first people to report the importance of dipping pruning tools in a germicide to control fire blight. Keil and van der Zwet (1967) showed that there was an 80% transmission of E. amylovora during pruning when secateurs were not decontaminated. Trials completed in 1986 showed that one contaminated pruning shear from bacterial ooze could transmit the bacteria and cause infection in more than 300 cutting sites on apple shoots (Nachtigall et al. 1986).
Many growers do not believe that removal of fire blight cankers helps control fire blight. Instead, they believe pruning contributes to its spread within orchard blocks. If growers implement pruning, sterilisation of pruning tools is often considered nonessential or cumbersome. Reasons that contribute to this thinking are cost effectiveness to complete the job, bleaching of clothing and tool corrosion from sodium hypochlorite or flammability of various alcohols in use.
This research was carried out to assess various commercial sterilants that could be used to sterilise pruning tools without the issues of market access, metal corrosion, bleaching of clothing or the flammability risks.
Multiple trials were set up over 3 years to determine the efficacy of several commercial products to kill E. amylovora when misted onto secateurs. Subsequent years’ trials were built on the previous year’s results.
Materials and methods
Lab assays of sterilant to kill Erwinia amylovora
Inoculum production
E. amylovora isolate (ICMP 236) [International Collection of Micro-organisms from Plants (ICMP), Manaaki Whenua, Landcare Research New Zealand Ltd] inoculum solution was made on the morning of each trial. Inoculum was prepared by growing the bacteria on King’s B agar (King et al. 1954) at 26 °C. After 48 h, bacteria were harvested and suspended in a phosphate buffered saline solution (PBS), pH 7.2. The bacterial concentration was determined by a spectrophotometer, then verified by dilution plating. Inoculum was produced at a concentration of 1 × 106 cfu/mL.
Sterilant efficacy using poison plate assay
Sterilant solutions (Table 1) were trialled for effectiveness against E. amylovora in poison plate assays over three successive assays.
To test the efficacy of a sterilant to kill E. amylovora on secateurs, clean, sterile used secateurs (Lowe, Germany) were dipped into a beaker containing the E. amylovora inoculum for 4 s. The secateurs were removed from the inoculum, shaken to remove excess inoculum, then approximately 0.18 mL of one the various sterilant treatments (Table 1) was applied using a spray bottle until the secateur blade and anvil were completely coated. The sterilant was left on for 10 s to allow time for sterilant action, before the excess was removed by shaking. The blade edge and anvil of the secateurs were then swabbed with a dry sterile cotton swab and placed into a sterile Eppendorf tube containing 1.16 mL sterile distilled water. The end volume in each tube was approximately 1 mL as the swabs tended to absorb and retain the remainder of the solution.
Tubes containing the washings were vortexed to ensure mixing, then a 0.1 mL aliquot was used to make a tenfold dilution series down to 10−12. A 0.1 mL aliquot from each dilution was spread onto a King’s B agar plate using a glass cell spreader. Plates were incubated at 26 °C for 2–3 days and number of colony forming units (cfu) counted to determine concentration. Each sterilant treatment was replicated six times using six different secateurs. Secateurs were sterilised at the beginning and between sterilants by dipping into 95% ethanol and flaming.
Sterilant efficacy using infected plant material
Sterilants that killed all or most of E. amylovora inoculum in the lab-based assay were assessed using infected pear shoots that contained sticky bacterial ooze in year 2 and again in year 3. Six secateurs per treatment were cut into an infected pear shoot that contained sticky bacterial ooze and then subsequently sterilised by spraying a suspension of one of the test products onto the secateurs in year 2 or 3. The test sterilants used in year 2 were sterile distilled water, 70% ethanol, 80% ethanol, 95% ethanol, 10% Dettol, 50% Dettol, 0.1% HarvestCide® gel, 0.5% HarvestCide gel, 1% HarvestCide gel, 0.068% sodium hypochlorite, 0.135% sodium hypochlorite, 0.5% sodium hypochlorite, 1% sodium hypochlorite. The test sterilants used in year 3 were sterile distilled water, 80% ethanol, 1% Dettol, 10% Dettol, 50% Dettol, 0.5% sodium hypochlorite, 1% sodium hypochlorite, 1% Virkon™. After 10 s application, a swab was taken off the secateurs blade edge and anvil, suspended into 1 mL sterile distilled water and then 0.1 mL of the washing plated onto Kings B media. Plates were incubated at 26 °C for 2 days, then bacterial colony numbers were recorded.
Bleaching properties
Of each sterilant, 0.1 mL was placed onto a black woven square of cotton fabric, and any colour bleaching of the dyes was observed.
Metal corrosion
Corrosivity of each sterilant was assessed using GEM stainless steel razor or scalpel blades of varying age and condition. These were thoroughly scrubbed to abrade off any surface coating that might interfere with assessments, and to remove any pre-existing markings. All blades were mixed together and selected at random for each trial to ensure consistency in starting condition across treatment groups. Six blades were then placed in each of the trial sterilant baths for 15 min to facilitate enough corrosion to compare between treatments. The blades were then drained and left exposed to the atmosphere for 4 days, before each blade was ranked on levels of observable corrosion. The rankings were as follows: 0 = no evident corrosion, 1 = mottled discolouration, 2 = severe corrosion/discolouration/flecks of rust, 3 = obvious corrosion or rust.
Data analysis
Data were analysed using a mixed effects model, with fixed effects for sterilant and inoculum, and random effects for trial, trial × rep and trial × sterilant × inoculum.
Results
Lab assays of sterilant to kill Erwinia amylovora
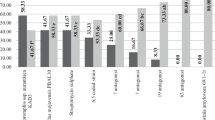
Most of the trialed sterilants were effective at reducing the E. amylovora, in comparison to the control, within the 10 s time frame following sterilant application (Sterilant effect F = 14.9 on 26 and 11 DF, p < 0.001). Methylated spirits (80%), sodium hypochlorite (0.5%, 0.135% and 0.068%), Bac-Stop (1%, 2%), Dettol (1% and 2%) and HarvestCide gel (1%) were all found to be effective in killing E. amylovora on contaminated secateurs (Table 1).
Bleaching properties
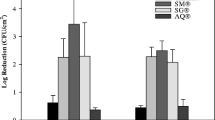
The majority of sterilants tested were not found to visibly bleach dyed fabric. Sodium hypochlorite was found to visibly bleach fabric within 30 min of exposure. The 1% solution exhibited higher bleaching capacity than did the 0.5% solution (Fig. 1). HarvestCide gel caused a very slight almost negligible bleaching. None of the other sterilant treatments caused damage to the cloth fabric, suggesting that there would not be any issue of clothing damage if used by orchard workers to sterilise cutting tools.
Corrosion of tools by sterilants
The cumulative corrosion scores for the replicate blade samples were determined. Bac-Stop, HarvestCide gel (Fig. 2), Virkon and sodium hypochlorite caused corrosion of metal blades. No other products tested did not cause degradation of metal.
Sterilant efficacy using infected plant material
Year 2
Candidate sterilants from the first trial were re-tested using infected plant material. No E. amylovora colonies grew from the blade and anvil washings following the 1% HarvestCide gel treatment (Table 2). All other treatments significantly reduced the bacteria concentration (p = 0.001) on the secateurs when compared to the positive control. However, some treatments were significantly better than others with HarvestCide gel and Dettol giving the best kill.
Year 3
Within year 3’s trial, the best performing sterilant when trialing using bacterial ooze in infected plants, was 0.5% and 1% sodium hypochlorite (Table 3) which resulted in a complete bacterial kill. Statistically similar was Dettol (1%, 10%, 50%) and 80% ethanol, which provided a good but not complete kill rate.
Discussion and conclusions
Overall, the best products for secateur sterilisation based on laboratory-produced inoculum and tests were 80% methylated spirits, sodium hypochlorite, Virkon, HarvestCide gel, Bac-Stop and Dettol.
For effective hygiene, a sterilant that provides a 100% kill (1% sodium hypochlorite) would be preferable to one that does not give a complete kill. Secateurs that were treated with each of the trial’s sterilants had a significantly lower bacteria count following blade and anvil washings compared to the untreated positive control (Table 3). There were no significant differences between the sterilants, with the exception of Virkon which did not kill as much bacteria as 0.05% or 1% sodium hypochlorite. There were no significant differences between the other sterilants trialed.
Dettol antiseptic, whilst not giving a 100% kill, did work well with infected plant material with bacterial ooze. It was a pleasant product to work with in the field, did not cause bleaching of clothes, is not flammable and does not cause corrosion of tools. A good bacterial kill was observed at a Dettol concentration of 10% and above. Dettol is a common disinfectant and antiseptic introduced in 1932 by the British company Reckitt. In Germany it is sold under the name Sagrotan. The active ingredients of Dettol antiseptic are chloroxylenol, propan-2-ol and α-terpineol. Chloroxylenol is used in hospitals and households for disinfection and sanitation. It is also commonly used in antibacterial soaps, wound-cleansing applications and household antiseptics (Bruch 1995). It is thought to act by disrupting microbial cell walls and inactivating cellular enzymes (Bednarek et al. 2021). Α-terpineol is a common ingredient in commercial disinfectants that can inhibit biofilm formation of Staphylococcus aureus (Vazquez-Sanchez et al. 2018) so it may have the same effect on inhibiting biofilm in E. amylovora. Propan-2-ol has been shown to be an effective hand disinfectant along with propan-1-ol and ethanol. 80% ethanol was shown to be the optimal concentration of ethanol for decontamination but was not as effective as propan-2-ol (iso-propanol) (Rotter et al. 1977). The use of Dettol would be suitable for most international organic requirements. We found that 1% sodium hypochlorite gave good bacterial kill and meets the requirements for use in organic standards. Similarly, Californian researchers also found that a 1:5 dilution of Clorox (sodium hypochlorite, 5.25%) prevented the transmission of E. amylovora (Teviotdale et al. 1991) as did Keil and van der Zwet in 1967. However, it is very damaging to pruning tools and causes unsightly bleaching of clothing.
Bac-Stop, whose active ingredient is benzalkonium chloride, gave good bacterial kill, but is not suitable for use in organic production as it persists on surfaces after a standard intervening event (e.g. rinse down) so does not meet New Zealand’s BioGro organic standard (Organic Certification Programmes | Organic Certification NZ | Organic Experts NZ (biogro.co.nz)).
One percent HarvestCide gel, whose active ingredient is 1-bromo-3-chloro-5,5-dimethylhydantoin, gave 100% bacterial kill when pruning fire blight infected shoots and caused little or no bleaching to clothing. However, like sodium hypochlorite, it caused corrosion to metal.
Another good sterilant was 80% ethanol (methylated spirit) which significantly reduced the bacterial population when pruning fire blight infected shoots. However, the product is highly flammable and evaporates quickly under ambient conditions. The flammability of methylated spirts presents a health and safety risk for personnel with various potential ignition sources in the work environment during storage, transport or use. Caution would also need to be taken with its storage and use to ensure that the concentration does not decline before use due to evaporation.
Virkon was not as effective in killing E. amylovora as sodium hypochlorite but has advantages of not bleaching clothing and is not flammable. However, it did cause corrosion of tools. The active ingredients are pentapotassium bis(peroxymonosulphate) bis(sulphate), polyphosphoric acid, benzenesulfonic acid, malic acid, sulphamidic acid, sodium chloride, sodium toluenesulphonate, potassium hydrogensulphate, dipotassium peroxodisulphate and dipentene. It has a colour indicator to show when the product still has a sterilisation capacity which ensures that bacterial kill can be maintained.
A small number of Pantoea agglomerans colonies were observed in the washings without a sterilant but were absent from the washings from any of the sterilant treated secateurs. This suggests that P. agglomerans may be quite sensitive to a range of various sterilants.
The findings from these studies provides four suitable sterilants for use whilst pruning in both organic and conventional production to control fire blight. Taking into consideration the health and safety aspect of flammable ethanol, damage to clothing from the bleaching by the sodium hypochlorite, and corrosion of tools, our recommendation would be Dettol for secateur sterilisation as it does not cause clothing damage or corrosion of tools, is nonflammable and pleasant to use.
References
Bednarek RS, Nassereddin A, Ramsey ML (2021) Skin antiseptics. In: StatPearls. StatPearls. PMID 29939630. https://www.ncbi.nlm.nih.gov/books/NBK507853/#article-29087.s10
Bruch MK (1995) Chloroxylenol: an old-new antimicrobial. In: Ascenzi JM (ed) Handbook of disinfectants and antiseptics. CRC Press, Boca Raton, pp 265–291
Burrill TJ (1882) The bacteria: an account of their nature and effects, together with a systematic description of the species. Illinois Indust Univ 11th Rep, pp 93–157
Keil H, van der Zwet T (1967) Sodium hypochlorite as a disinfectant of pruning tools for fire blight control. Plant Dis Rep 51:753–755
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Nachtigall M, Ficke W, Schaeffer H (1986) Feuerbrand-bekaempfung an apfel durch Schnittmassnahmen. Gartenbau 33:7–9
Rotter M, Koler W, Kundi M (1977) Usability of three alcohols for a standard disinfection method to be employed for the evaluation of procedures for the hygienic disinfection of hands. Zentralbl Bakteriol Orig 164:428–438
Teviotdale B, Wiley M, Harper D (1991) How disinfectants compare in preventing transmission of fire blight. Calif Agric 45:21–23
Van der Zwet T, Orolaza-Halbrendt N, Zeller W (2012) Fire blight: history, biology, and management. APS Press. Am Phytopathol Soc, St. Paul MN, USA, 421 pp. ISBN 978-0-89054-394-8
Vazquez-Sanchez D, Galvao JA, Mazine MR, Gloria EM, Oetterer M (2018) Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int J Food Microbiol 286:128–138
Winslow CEA, Broadhurst J, Buchanan RE, Krumwiede Jr C, Rogers LA, Smith GH (1920) The families and genera of the bacteria. Final report of the Committee of the Society of American Bacteriologists on characterization and classification of bacterial types. J Bacteriol 5:191–229
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was funded by New Zealand Apples and Pears Inc. and the New Zealand Sustainable Food and Fibre Futures Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest regarding this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horner, M., Newland, J. & McCourt, T. Efficacy of sterilants to kill Erwinia amylovora. J Plant Pathol (2024). https://doi.org/10.1007/s42161-023-01584-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-023-01584-x