Abstract
The proteins and polysaccharides are always remained in the effluent of biotreated process, which leads to further pollution for advanced treatment or aqueous environment. In this study, Fe3O4/polynailine composite (Fe3O4@PANI) was prepared by means of in situ surface polymerization, and was used as the adsorbent for removal of proteins and polysaccharides from biotreated wastewater. The removal rate of polysaccharide by PANI (69.43%) was higher than that by Fe3O4@PANI (61.33%), while the removal percentage of protein by Fe3O4@PANI (65.25%) was higher than that by original PANI (47.56%). The adsorption process was fitted well with the quasi-first-order kinetic and Langmuir isotherm models. Finally, it was determined that electrostatic attraction is the primary mechanism for the adsorption of proteins and polysaccharides by PANI-based materials.
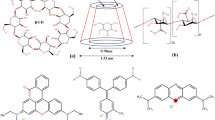
Graphical abstract
The Fe3O4/polyaniline can significantly remove proteins and polysaccharides from bio-treated wastewater due to its positively charged surface.










Similar content being viewed by others
References
Wainaina S et al (2020) Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour Technol 301:122778. https://doi.org/10.1016/j.biortech.2020.122778
Li LL, Tong ZH, Fang CY, Chu J, Yu HQ (2015) Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res 70:1–8. https://doi.org/10.1016/j.watres.2014.11.042
Benyahia B, Sari T, Harmand J, Cherki B (2011) Modeling of the soluble microbial products (SMP) in anaerobic membrane bioreactors (AMBR): equilibria and stability of the AM2b model. IFAC Proc 44:3789–3794. https://doi.org/10.3182/20110828-6-IT-1002.01195
Lee DY, Li YY, Noike T, Cha GC (2008) Behavior of extracellular polymers and bio-fouling during hydrogen fermentation with a membrane bioreactor. J Membr Sci 322:13–18. https://doi.org/10.1016/j.memsci.2008.04.031
Wu M, Liang Y, Peng H, Ye J, Wu J, Shi W, Liu W (2019) Bioavailability of soluble microbial products as the autochthonous precursors of disinfection by-products in aerobic and anoxic surface water. Sci Total Environ 649:960–968. https://doi.org/10.1016/j.scitotenv.2018.08.354
Wisniewski C, Grasmick A (1998) Floc size distribution in a membrane bioreactor and consequences for membrane fouling. Colloid Surface A 138:403–411. https://doi.org/10.1016/S0927-7757(96)03898-8
Sunny W, Greg G, Hoek Eric MV (2005) Direct observation of microbial adhesion to membranes. Environ Sci Technol 39:6461–6469. https://doi.org/10.1021/es050188s
Meng PC, Peiying H, Huiling G, Wen TL (2005) Biofilm formation characteristics of bacterial isolates retrieved from a reverse osmosis membrane. Environ Sci Technol 39:7541–7550. https://doi.org/10.1021/es050170h
Chen MY, Lee DJ, Tay J (2006) Extracellular polymeric substances in fouling layer. Sep Sci Technol 41:1467–1474. https://doi.org/10.1080/01496390600683597
Ramesh A, Lee D, Wang M, Hsu J, Juang R, Hwang K, Liu J, Tseng S (2006) Biofouling in membrane bioreactor. Sep Sci Technol 41:1345–1370. https://doi.org/10.1080/01496390600633782
Visvanathan C, Abeynayaka A (2012) Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr Water Treat 3:1–23. https://doi.org/10.12989/mwt.2012.3.1.001
Zhang W, Cao B, Wang D, Ma T, Xia H, Yu D (2016) Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS). Water Res 88:728–739. https://doi.org/10.1016/j.watres.2015.10.049
Davis JA (1982) Adsorption of natural dissolved organic matter at the oxide/water interface. Geochim Cosmochim Acta 46:2381–2393. https://doi.org/10.1016/0016-7037(82)90209-5
Kim J, Kim K, Ye H, Lee E, Shin C, McCarty PL, Bae J (2011) Anaerobic fluidized bed membrane bioreactor for wastewater treatment. Environ Sci Technol 45:576–581. https://doi.org/10.1021/es1027103
Chen L, Cheng P, Ye L, Chen H, Xu X, Zhu L (2020) Biological performance and fouling mitigation in the biochar-amended anaerobic membrane bioreactor (AnMBR) treating pharmaceutical wastewater. Bioresource Technol 302:122805. https://doi.org/10.1016/j.biortech.2020.122805
Luo X, Yang G, Schubert DW (2021) Electrically conductive polymer composite containing hybrid graphene nanoplatelets and carbon nanotubes: synergistic effect and tunable conductivity anisotropy. Adv Compos Hybrid Ma. https://doi.org/10.1007/s42114-021-00332-y
Nguyen TD, Nguyen TA, Pham M, Piro B, Normand B, Takenouti H (2004) Mechanism for protection of iron corrosion by an intrinsically electronic conducting polymer. J Electroanal Chem 572:225–234. https://doi.org/10.1016/j.jelechem.2003.09.028
Bleda-Martínez MJ, Morallon E, Cazorla-Amorós D (2007) Polyaniline/porous carbon electrodes by chemical polymerisation: effect of carbon surface chemistry. Electrochim Acta 52:4962–4968. https://doi.org/10.1016/j.electacta.2007.01.073
Humpolíček P et al (2015) Stem cell differentiation on conducting polyaniline. RSC Adv 5:68796–68805. https://doi.org/10.1039/C5RA12218J
Lü Z, Xiujuan X, Biao P, Guofeng F (2016) Extracellular polymeric substance (EPS) characteristics and comparison of suspended and attached activated sludge at low temperatures. J Thu (Sci Technol) 56:1009–1015. https://doi.org/10.16511/j.cnki.qhdxxb.2016.21.051
Zhang F, Cui W, Wang B, Xu B, Liu X, Liu X, Jia Z, Wu G (2021) Morphology-control synthesis of polyaniline decorative porous carbon with remarkable electromagnetic wave absorption capabilities. Compos Part B-Eng 204:108491. https://doi.org/10.1016/j.compositesb.2020.108491
Ingle R V, Shaikh S F, Bhujbal P K, Pathan H M, Tabhane VA (2020) Polyaniline doped with protonic acids: optical and morphological studies. ES Mater Mfg 8:54–59. https://doi.org/10.30919/esmm5f732
Hu Q, Zhou J, Qiu B, Wang Q, Song G, Guo Z (2021) Synergistically improved methane production from anaerobic wastewater treatment by iron/polyaniline composite. Adv Compos Hybrid Ma 2:265–273. https://doi.org/10.1021/acssuschemeng.8b05847
Liu S, Liu X, Li Z, Yang S, Wang J (2011) Fabrication of free-standing graphene/polyaniline nanofibers composite paper via electrostatic adsorption for electrochemical supercapacitors. New J Chem 35:369–374. https://doi.org/10.1039/C0NJ00718H
Liao Y, Wang Y, Ouyang L, Dong Y, Zhou J, Hu Q, Qiu B (2021) Conductive polyaniline enhanced decolorization of azo dyes in anaerobic wastewater treatment. FAF 6: 35–42. https://doi.org/10.30919/esfaf584
Li S et al (2019) Facile synthesis of nanostructured polyaniline in ionic liquids for high solubility and enhanced electrochemical properties. Adv Compos Hybrid Ma 2:279–288. https://doi.org/10.1007/s42114-019-00103-w
Guo J, Chen Z, Abdul W, Kong J, Khan MA, Young DP, Zhu J, Guo Z (2021) Tunable positive magnetoresistance of magnetic polyaniline nanocomposites. Adv Compos Hybrid Ma 4:534–542. https://doi.org/10.1007/s42114-021-00242-z
Shao Y, Bai H, Wang H, Fei G, Li L, Zhu Y (2021) Magnetically sensitive and high template affinity surface imprinted polymer prepared using porous TiO2-coated magnetite-silica nanoparticles for efficient removal of tetrabromobisphenol A from polluted water. Adv Compos Hybrid Ma. https://doi.org/10.1007/s42114-021-00361-7
Dwivedi M (2018) Exopolysaccharide (EPS) producing isolates from sugarcane field soil and antibacterial activity of extracted EPSs. Acta Scientific Microbiology 1:06–13. https://doi.org/10.31080/ASMI.2018.01.0032
Felz S, Vermeulen P, Loosdrecht MC, Lin YM (2019) Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res 157:201–208. https://doi.org/10.1016/j.watres.2019.03.068
Kang E, Ting Y, Neoh K, Tan K (1995) Electroless recovery of precious metals from acid solutions by N-containing electroactive polymers. Synthetic Met 69:477–478. https://doi.org/10.1016/0379-6779(94)02533-5
Neoh KG, Pun MY, Kang ET, Tan KL (1995) Polyaniline treated with organic acids: doping characteristics and stability. Synthetic Met 73:209–215. https://doi.org/10.1016/0379-6779(95)80018-2
Chiang JC, MacDiarmid AG (1986) ‘Polyaniline’: protonic acid doping of the emeraldine form to the metallic regime. Synthetic Met 13:193–205. https://doi.org/10.1016/0379-6779(86)90070-6
Kassahun K et al (2001) Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem Res Toxicol 14:62–70. https://doi.org/10.1021/tx000180q
Wei Y, Luo W, Zhuang Z, Dai B, Ding J, Li T, Ma M, Yin X, Ma Y (2021) Fabrication of ternary MXene/MnO2/polyaniline nanostructure with good electrochemical performances. Adv Compos Hybrid Ma 4:1082–1091. https://doi.org/10.1007/s42114-021-00323-z
Arasi AY, Jeyakumari JJL, Sundaresan B, Dhanalakshmi V, Anbarasan R (2009) The structural properties of poly (aniline)—analysis via FTIR spectroscopy. Spectrochim Acta A 74:1229–1234. https://doi.org/10.1016/j.saa.2009.09.042
Trchová M, Šeděnková I, Tobolková E, Stejskal J (2004) FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym Degrad Stabil 86: 179–185. https://doi.org/10.1016/j.polymdegradstab.2004.04.011
Du J et al (2020) Boosting the utilization and electrochemical performances of polyaniline by forming a binder-free nanoscale coaxially coated polyaniline/carbon nanotube/carbon fiber paper hierarchical 3D microstructure composite as a supercapacitor electrode. ACS Omega 5:22119–22130. https://doi.org/10.1021/acsomega.0c02151
Mostafaei A, Zolriasatein A (2012) Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog Nat Sci Mater 22:273–280. https://doi.org/10.1016/j.pnsc.2012.07.002
Gupta P, Dillon AC, Bracker AS, George SM (1991) FTIR studies of H2O and D2O decomposition on porous silicon surfaces. Surf Sci Lett 245:360–372. https://doi.org/10.1016/0039-6028(91)90038-T
Zhuang Z, Wang W, Wei Y, Li T, Ma M, Ma Y (2021) Preparation of polyaniline nanorods/manganese dioxide nanoflowers core/shell nanostructure and investigation of electrochemical performances. Adv Compos Hybrid Ma 4:938–945. https://doi.org/10.1007/s42114-021-00225-0
Nicolet Y, Lacey AL, Vernède X, Fernandez VM, Hatchikian EC, Fontecilla Camps JC (2001) Crystallographic and FTIR spectroscopic evidence of changes in Fe coordination upon reduction of the active site of the Fe-only hydrogenase from Desulfovibrio desulfuricans. J Am Chem Soc 123:1596–1601. https://doi.org/10.1021/ja0020963
Liu S, Yu B, Wang S, Shen Y, Cong H (2020) Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv Colloid Interface Sci 281:102165. https://doi.org/10.1016/j.cis.2020.102165
Liu B, Wang D, Yu G, Meng X (2013) Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives - a review. J Ocean Univ China 12:500–508. https://doi.org/10.1007/s11802-013-2113-0
Yahyaei M, Mehrnejad F, Naderi MH, Rezayan AH (2018) Protein adsorption onto polysaccharides: comparison of chitosan and chitin polymers. Carbohydr Polyme 191:191–197. https://doi.org/10.1016/j.carbpol.2018.03.034
Liu B, Wang D, Yu G, Meng X (2013) Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives - a review. J Ocean Univ China 12:500–508. https://doi.org/10.1007/s11802-013-2113-0
Huang W, He H, Dong B, Chu H, Xu G, Yan Z (2015) Effects of macro-porous anion exchange and coagulation treatment on organic removal and membrane fouling reduction in water treatment. Desalination 355:204–216. https://doi.org/10.1016/j.desal.2014.10.045
Feng Z, Shao Z, Yao J, Huang Y, Chen X (2009) Protein adsorption and separation with chitosan-based amphoteric membranes. Polymer 50:1257–1263. https://doi.org/10.1016/j.polymer.2008.12.046
Shi Y, Liu T, Han Y, Zhu X, Zhao X, Ma X, Jiang D, Zhang Q (2017) An efficient method for decoloration of polysaccharides from the sprouts of Toona sinensis (A. Juss.) Roem by anion exchange macroporous resins. Food chem 217:461–468. https://doi.org/10.1016/j.foodchem.2016.08.079
Yang R, Meng D, Song Y, Li J, Zhang Y, Hu X, Ni Y, Li Q (2012) Simultaneous decoloration and deproteinization of crude polysaccharide from pumpkin residues by cross-linked polystyrene macroporous resin. J Agric Food chem 60:8450–8456. https://doi.org/10.1021/jf3031315
Yang X, Yi H, Tang X, Zhao S, Yang Z, Ma Y, Feng T, Cui X (2018) Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J Environ Sci 67:104–114. https://doi.org/10.1016/j.jes.2017.06.032
Yang W, Yu Z, Pan B, Lv L, Zhang W (2015) Simultaneous organic/inorganic removal from water using a new nanocomposite adsorbent: a case study of p-nitrophenol and phosphate. Chem Eng J 268:399–407. https://doi.org/10.1016/j.cej.2015.01.051
Craik AD, Leibovich S (1976) A rational model for Langmuir circulations. J Fluid Mech 73:401–426. https://doi.org/10.1017/S0022112076001420
Dragan ES, Humelnicu D, Dinu MV, Olariu RI (2017) Kinetics, equilibrium modeling, and thermodynamics on removal of Cr (VI) ions from aqueous solution using novel composites with strong base anion exchanger microspheres embedded into chitosan/poly (vinyl amine) cryogels. Chem Eng J 330:675–691. https://doi.org/10.1016/j.cej.2017.08.004
Meng F, Zhou Z, Ni BJ, Zheng X, Huang G, Jia X, Li S, Xiong Y, Kraume M (2011) Characterization of the size-fractionated biomacromolecules: tracking their role and fate in a membrane bioreactor. Water Res 45:4661–4671. https://doi.org/10.1016/j.watres.2011.06.026
Funding
This work is supported by the Fundamental Research Funds for the Central Universities (2021ZY77). The authors also extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number “x_2020_IF.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Huang, W., Qiu, B. et al. Effective removal of proteins and polysaccharides from biotreated wastewater by polyaniline composites. Adv Compos Hybrid Mater 5, 1888–1898 (2022). https://doi.org/10.1007/s42114-022-00508-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-022-00508-0