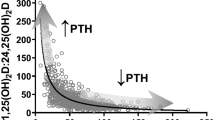
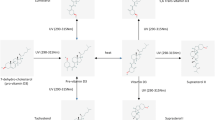
Abstract
Today, the possibility exists to measure a number of different vitamin D metabolites with accurate and precise methods. The most abundant vitamin D metabolite, 25(OH)D, is considered the best marker for estimating vitamin D status and is therefore the most commonly measured in clinical practice. There is no consensus on the added value of measuring other metabolites beyond 25-hydroxyvitamin D, although, in some special clinical scenarios and complicated cases, these metabolites may provide just the information needed for an accurate diagnosis. The problem this review addresses is which metabolite to measure and when and how to measure it.
Similar content being viewed by others
Abbreviations
- 7-DHC:
-
Dehydrocholesterol or provitamin D3)
- 24,25(OH)2D3:
-
24,25-Dihydroxyvitamin D3
- 25(OH)D3:
-
Calcidiol or 25-hydroxyvitamin D3
- 1α,25(OH)2D3:
-
Calcitriol or 1,25-dihydroxyvitamin D3
- C3-epi-25(OH)D:
-
C3-epimer of 25(OH)D
- CYP27B1:
-
25(OH)D-1a-hydroxylase
- CYP24A1:
-
24-Hydroxylase
- PTH:
-
Parathyroid hormone
- FGF-23:
-
Fibroblast growth factor-23
- VDBP:
-
Vitamin D-binding protein
- VDDR:
-
Vitamin D-dependent rickets
- VDR:
-
Vitamin D receptor
- VDSP:
-
Vitamin D standardization program
- VMR:
-
Vitamin D metabolic ratio
- CLD:
-
Chronic liver disease
- CKD:
-
Chronic kidney disease
- SHPT:
-
Secondary hyperparathyroidism
- UV:
-
Ultraviolet
- HPLC:
-
High-performance liquid chromatography
- UHPLC:
-
Ultra-high-pressure liquid chromatography
- LC-MS/MS:
-
Liquid chromatography coupled with mass spectrometry
- ID LC-MS/MS:
-
Isotope dilution liquid chromatography coupled with mass spectrometry
- CPBA:
-
Competitive protein binding assays
- RIA:
-
Radioimmunoassay
- ELISA:
-
Enzyme-linked immunosorbent assays
- CLIA:
-
Chemiluminescent assays
- RMP:
-
Reference measurement procedure
- NIST:
-
National Institute for Standards and Technology
- IFCC:
-
International Federation of Clinical Chemistry
- JCTLM:
-
Joint Committee for Traceability in Laboratory Medicine
- AACC:
-
American Association for Clinical Chemistry
- DEQAS:
-
Vitamin D External Quality Assessment Scheme
- CAP:
-
College of American Pathologists
- CDC:
-
Center for Disease Control
- EQA:
-
External quality assessment
References
Bikle DD, Vitamin D (2018) Assays. Front Horm Res 50:14–30
Byrdwell WC, Devries J, Exler J, Harnly JM, Holden JM, Holick MF et al (2008) Analyzing vitamin D in foods and supplements: methodologic challenges. Am J Clin Nutr 88(2):554S–557S
Cai SS, Syage JA (2006) Comparison of atmospheric pressure photoionization, atmospheric pressure chemical ionization, and electrospray ionization mass spectrometry for analysis of lipids. Anal Chem 78(4):1191–1199
Adamec J, Jannasch A, Huang J, Hohman E, Fleet JC, Peacock M et al (2011) Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2 , vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J Sep Sci 34(1):11–20
Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E (2017) Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med 55(1):3–26
Heureux N, Vitamin D (2017) Testing-where are we and what is on the horizon? Adv Clin Chem 78:59–101
Hoofnagle AN, Wener MH (2009) The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods 347(1–2):3–11
Duan X, Weinstock-Guttman B, Wang H, Bang E, Li J, Ramanathan M et al (2010) Ultrasensitive quantification of serum vitamin D metabolites using selective solid-phase extraction coupled to microflow liquid chromatography and isotope-dilution mass spectrometry. Anal Chem 82(6):2488–2497
Higashi T, Shimada K, Toyo'oka T (2010) Advances in determination of vitamin D related compounds in biological samples using liquid chromatography-mass spectrometry: a review. J Chromatogr B Anal Technol Biomed Life Sci 878(20):1654–1661
El-Khoury JM, Reineks EZ, Wang S (2011) Progress of liquid chromatography-mass spectrometry in measurement of vitamin D metabolites and analogues. Clin Biochem 44(1):66–76
Valcour A, Zierold C, Podgorski AL, Olson GT, Wall JV, DeLuca HF et al (2016) A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples. J Steroid Biochem Mol Biol 164:120–126
Mahlow J, Bunch DR, Wang S (2016) Quantification of 1,25-dihydroxyvitamin D2 and D3 in serum using liquid chromatography-tandem mass spectrometry. Methods Mol Biol 1378:291–300
Wan D, Yang J, Barnych B, Hwang SH, Lee KS, Cui Y et al (2017) A new sensitive LC/MS/MS analysis of vitamin D metabolites using a click derivatization reagent, 2-nitrosopyridine. J Lipid Res 58(4):798–808
Carter GD, Berry J, Durazo-Arvizu R, Gunter E, Jones G, Jones J et al (2017) Quality assessment of vitamin D metabolite assays used by clinical and research laboratories. J Steroid Biochem Mol Biol 173:100–104
Zittermann A, Ernst JB, Becker T, Dreier J, Knabbe C, Gummert JF et al (2016) Measurement of circulating 1,25-dihydroxyvitamin D: comparison of an automated method with a liquid chromatography tandem mass spectrometry method. Int J Anal Chem 2016:8501435
Souberbielle JC, Cavalier E, Delanaye P, Massart C, Brailly-Tabard S, Cormier C et al (2015) Serum calcitriol concentrations measured with a new direct automated assay in a large population of adult healthy subjects and in various clinical situations. Clin Chim Acta 451(Pt B):149–153
Dirks NF, Martens F, Vanderschueren D, Billen J, Pauwels S, Ackermans MT et al (2016) Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC-MS/MS method. J Steroid Biochem Mol Biol 164:127–133
Lips P (2007) Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 22(11):1668–1671
Dusso AS (2011) Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int Suppl (2011) 1(4):136–141
Dirks NF, Ackermans MT, Lips P, de Jongh RT, Vervloet MG, de Jonge R et al (2018) The when, what & how of measuring vitamin D metabolism in clinical medicine. Nutrients 10(4):482. https://doi.org/10.3390/nu10040482
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
Johal M, Levin A (2009) Vitamin D and parathyroid hormone in general populations: understandings in 2009 and applications to chronic kidney disease. Clin J Am Soc Nephrol 4(9):1508–1514
Zalewski A, Ma NS, Legeza B, Renthal N, Flück CE, Pandey AV (2016) Vitamin D-dependent rickets type 1 caused by mutations in CYP27B1 affecting protein interactions with adrenodoxin. J Clin Endocrinol Metab 101(9):3409–3418
Molin A, Wiedemann A, Demers N, Kaufmann M, Do Cao J, Mainard L et al (2017) Vitamin D–dependent rickets type 1B (25-hydroxylase deficiency): a rare condition or a misdiagnosed condition? J Bone Miner Res 32:1893–1899. https://doi.org/10.1002/jbmr.3181
Thacher TD, Levine MA (2017) CYP2R1 mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol 173:333–336
Carpenter TO (2012) The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 30(1):1–9
Kinoshita Y, Fukumoto S (2018) X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr Rev 39(3):274–291
Malloy PJ, Feldman D (2010) Genetic disorders and defects in vitamin d action. Endocrinol Metab Clin N Am 39(2):333–346 table of contents
Adams JS, Hewison M (2012) Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 523(1):95–102
Donovan PJ, Sundac L, Pretorius CJ, d'Emden MC, McLeod DSA (2013) Calcitriol-mediated Hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab 98(10):4023–4029
Makris K, Sempos C, Cavalier E (2020) The measurement of vitamin D metabolites: part I—metabolism of vitamin D and the measurement of 25-hydroxyvitamin D. Hormones. https://doi.org/10.1007/s42000-019-00169-7
Jones G, Prosser DE, Kaufmann M (2014) Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55(1):13–31
Petkovich M, Jones G (2011) CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens 20(4):337–344
Leeuwenkamp OR, van der Wiel HE, Lips P, van der Vijgh WJ, Barto R, Greuter H et al (1993) Human pharmacokinetics of orally administered (24 R)-hydroxycalcidiol. Eur J Clin Chem Clin Biochem 31(7):419–426
Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B et al (2012) The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 82(6):693–700
Pike JW, Meyer MB (2012) Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch Biochem Biophys 523(1):2–8
de Boer IH, Sachs MC, Chonchol M, Himmelfarb J, Hoofnagle AN, Ix JH et al (2014) Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis 64(2):187–197
Berg AH, Powe CE, Evans MK, Wenger J, Ortiz G, Zonderman AB et al (2015) 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin Chem 61(6):877–884
Bosworth C, de Boer IH (2013) Impaired vitamin D metabolism in CKD. Semin Nephrol 33(2):158–168
Ketha H, Kumar R, Singh RJ (2016) LC-MS/MS for identifying patients with CYP24A1 mutations. Clin Chem 62(1):236–242
Carpenter TO (2017) CYP24A1 loss of function: clinical phenotype of monoallelic and biallelic mutations. J Steroid Biochem Mol Biol 173:337–340
Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y et al (2012) Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab 97(3):E423–E4E7
Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U et al (2011) Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365(5):410–421
Jacobs TP, Kaufman M, Jones G, Kumar R, Schlingmann K-P, Shapses S et al (2014) A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab 99(3):708–712
Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M et al (2015) Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 61(4):636–645
Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L et al (2011) The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol 126(3–5):72–77
Kaufmann M, Gallagher JC, Peacock M, Schlingmann K-P, Konrad M, DeLuca HF et al (2014) Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab 99(7):2567–2574
Ketha H, Thacher TD, Oberhelman SS, Fischer PR, Singh RJ, Kumar R (2018) Comparison of the effect of daily versus bolus dose maternal vitamin D(3) supplementation on the 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) ratio. Bone. 110:321–325
Molin A, Baudoin R, Kaufmann M, Souberbielle JC, Ryckewaert A, Vantyghem MC et al (2015) CYP24A1 mutations in a cohort of hypercalcemic patients: evidence for a recessive trait. J Clin Endocrinol Metab 100(10):E1343–E1E52
Selamet U, Katz R, Ginsberg C, Rifkin DE, Fried LF, Kritchevsky SB et al (2018) Serum calcitriol concentrations and kidney function decline, heart failure, and mortality in elderly community-living adults: the health, aging, and body composition study. Am J Kidney Dis 72(3):419–428
Tang JCY, Nicholls H, Piec I, Washbourne CJ, Dutton JJ, Jackson S et al (2017) Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J Nutr Biochem 46:21–29
Fabregat-Cabello N, Farre-Segura J, Huyghebaert L, Peeters S, Le Goff C, Souberbielle J-C et al (2017) A fast and simple method for simultaneous measurements of 25(OH)D, 24,25(OH)2D and the vitamin D metabolite ratio (VMR) in serum samples by LC-MS/MS. Clin Chim Acta 473:116–123
Jones G, Kaufmann M (2016) Vitamin D metabolite profiling using liquid chromatography–tandem mass spectrometry (LC–MS/MS). J Steroid Biochem Mol Biol 164:110–114
Dirks NF, Ackermans MT, de Jonge R, Heijboer AC (2019) Reference values for 24,25-dihydroxyvitamin D and the 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio. Clin Chem Lab Med 57(10):e259–e261. https://doi.org/10.1515/cclm-2018-1096
Tai SS, Nelson MA (2015) Candidate reference measurement procedure for the determination of (24R),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 87(15):7964–7970
Wise SA, Tai SS, Nelson MA, Burdette CQ, Camara JE, Hoofnagle AN et al (2017) Interlaboratory comparison for the determination of 24,25-dihydroxyvitamin D(3) in human serum using liquid chromatography with tandem mass spectrometry. J AOAC Int 100(5):1308–1317
Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE et al (2012) Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem 84(2):956–962
Bailey D, Veljkovic K, Yazdanpanah M, Adeli K (2013) Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin Biochem 46(3):190–196
Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M (2012) State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem 58(3):531–542
Farrell C, Soldo J, Williams P, Herrmann M (2012) 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med 50(11):1953–1963
Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M (2014) Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol 144(Pt A):132–137
Speeckaert M, Huang G, Delanghe JR, Taes YE (2006) Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 372(1–2):33–42
Bhan I (2014) Vitamin d binding protein and bone health. Int J Endocrinol 2014:561214
Adebanjo OA, Moonga BS, Haddad JG, Huang CL, Zaidi M (1998) A possible new role for vitamin D-binding protein in osteoclast control: inhibition of extracellular Ca2+ sensing at low physiological concentrations. Biochem Biophys Res Commun 249(3):668–671
Chun RF (2012) New perspectives on the vitamin D binding protein. Cell Biochem Funct 30(6):445–456
Bikle DD, Schwartz J (2019) Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol 10:317
Cooke NE, Haddad JG (1989) Vitamin D binding protein (Gc-globulin). Endocr Rev 10(3):294–307
Wang X, Shapses SA, Al-Hraishawi H (2017) Free and bioavailable 25-hydroxyvitamin D levels in patients with primary hyperparathyroidism. Endocr Pract 23(1):66–71
Bjorkhem-Bergman L, Torefalk E, Ekstrom L, Bergman P (2018) Vitamin D binding protein is not affected by high-dose vitamin D supplementation: a post hoc analysis of a randomised, placebo-controlled study. BMC Res Notes 11(1):619
Arnaud J, Constans J (1993) Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet 92(2):183–188
Lauridsen AL, Vestergaard P, Nexo E (2001) Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem 47(4):753–756
Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P (1981) Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest 67(3):589–596
Bikle DD, Gee E, Halloran B, Haddad JG (1984) Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 74(6):1966–1971
Mendel CM (1989) The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 10(3):232–274
Vieth R (1994) Simple method for determining specific binding capacity of vitamin D-binding protein and its use to calculate the concentration of "free" 1,25-dihydroxyvitamin D. Clin Chem 40(3):435–441
Chun RF, Nielson CM (2017) Free Vitamin D: Concepts, assays, outcomes and prospects. In: Feldman D, Wesley Pike J, Bouillon R, Giovannucci E, Goltzman D, Hewison M (eds) Vitamin D volume 1: biochemistry, physiology and diagnosis, vol 1, 4th edn. Elsevier
Schwartz JB, Lai J, Lizaola B, Kane L, Weyland P, Terrault NA et al (2014) Variability in free 25(OH) vitamin D levels in clinical populations. J Steroid Biochem Mol Biol 144(Pt A):156–158
Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA et al (2012) Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int 82(1):84–89
Tsuprykov O, Chen X, Hocher CF, Skoblo R, Lianghong Y, Hocher B (2018) Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol 180:87–104
Bikle DD, Siiteri PK, Ryzen E, Haddad JG (1985) Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 61(5):969–975
Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG (1986) Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest 78(3):748–752
Heureux N, Lindhout E, Swinkels L (2017) A direct assay for measuring free 25-hydroxyvitamin D. J AOAC Int 100(5):1318–1322
Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG (1986) Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 63(4):954–959
Feldman H, Rodbard D, Levine D (1972) Mathematical theory of cross-reactive radioimmunoassay and ligand-binding systems of equilibrium. Anal Biochem 45(2):530–556
Nielson CM, Jones KS, Bouillon R, Osteoporotic Fractures in Men Research G, Chun RF, Jacobs J et al (2016) Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations. N Engl J Med 374(17):1695–1696
Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M et al (2016) Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab 101(5):2226–2234
Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P et al (2014) A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab 99(5):1631–1637
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M et al (2013) Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 369(21):1991–2000
Bikle DD, Malmstroem S, Schwartz J (2017) Current controversies: are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol Metab Clin N Am 46(4):901–918
Hoofnagle AN, Eckfeldt JH, Lutsey PL (2015) Vitamin D-binding protein concentrations quantified by mass spectrometry. N Engl J Med 373(15):1480–1482
Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ et al (2016) Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res 31(6):1128–1136
Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH et al (2016) Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem 62(1):179–187
Acknowledgments
None of the authors declares any funding or grant related to this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makris, K., Sempos, C. & Cavalier, E. The measurement of vitamin D metabolites part II—the measurement of the various vitamin D metabolites. Hormones 19, 97–107 (2020). https://doi.org/10.1007/s42000-020-00188-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-020-00188-9