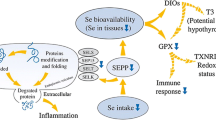
Abstract
Antibiotics are provided for infections caused by bacteria, and statins help to control hypercholesterolemia. When hungry, you need to eat, and when you are deficient in a particular nutrient, the diet should be chosen wisely to provide what is missing. In the matter of providing the essential trace element selenium (Se), there are two different but partly overlapping views on its nature and requirements. Some consider it a medication that should be given to a subset of more or less well-defined (thyroid) patients only, in order to alleviate symptoms, to improve the course of the disease or even to provide a cure, alone or in an adjuvant mode. Such treatment attempts are conducted for a short time period, and potential medical benefits and side effects are evaluated thoroughly. One could also approach Se in medicine in a more holistic way and evaluate primarily the nutritional status of the patient before considering supplementation. The available evidence for positive health effects of supplemental Se can be interpreted as the consequence of correcting deficiency instead of speculating on a direct pharmaceutical action. This short review provides a novel view on Se in (thyroid) disease and beyond and offers an alternative explanation for its positive health effects, i.e., its provision of the substrate needed for allowing adequate endogenous expression of those selenoproteins that are required in certain conditions. In Se deficiency, the lack of the trace element constitutes the main limitation for the required adaptation of selenoprotein expression to counteract health risks and alleviate disease symptoms. Supplemental Se lifts this restriction and enables the full endogenous response of selenoprotein expression. However, since Se does not act as a pharmacological medication per se, it should not be viewed as a dangerous drug, and, importantly, current data show that supplemental Se does not cause diabetes.


Similar content being viewed by others
References
Schwarz K, Foltz CM (1958) Factor 3 activity of selenium compounds. J Biol Chem 233:245–251
Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, Gladyshev VN, Hatfield DL et al (2016) Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 9:22–31. https://doi.org/10.1016/j.redox.2016.05.003
Suzuki KT, Ogra Y (2002) Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam 19:974–983. https://doi.org/10.1080/02652030210153578
Hoefig CS, Renko K, Kohrle J, Birringer M, Schomburg L (2011) Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J Nutr Biochem 22:945–955. https://doi.org/10.1016/j.jnutbio.2010.08.006
Santofimia-Castano P, Izquierdo-Alvarez A, De La Casa-Resino I, Martinez-Ruiz A, Perez-Lopez M, Portilla JC, Salido GM, Gonzalez A (2016) Ebselen alters cellular oxidative status and induces endoplasmic reticulum stress in rat hippocampal astrocytes. Toxicology 357-358:74–84. https://doi.org/10.1016/j.tox.2016.06.002
Bender KO, Garland M, Ferreyra JA, Hryckowian AJ, Child MA, Puri AW, Solow-Cordero DE, Higginbottom SK et al (2015) A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci Transl Med 7:306ra148. https://doi.org/10.1126/scitranslmed.aac9103
Sun GX, Van De Wiele T, Alava P, Tack FMG, Du Laing G (2017) Bioaccessibility of selenium from cooked rice as determined in a simulator of the human intestinal tract (SHIME). J Sci Food Agric 97:3540–3545. https://doi.org/10.1002/jsfa.8208
Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW (2006) Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomark Prev 15:804–810. https://doi.org/10.1158/1055-9965.epi-05-0950
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777. https://doi.org/10.1152/physrev.00039.2013
Turanov AA, Xu XM, Carlson BA, Yoo MH, Gladyshev VN, Hatfield DL (2011) Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis. Adv Nutr 2:122–128. https://doi.org/10.3945/an.110.000265
Diamond AM, Choi IS, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE et al (1993) Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec). J Biol Chem 268:14215–14223
Sengupta A, Carlson BA, Weaver JA, Novoselov SV, Fomenko DE, Gladyshev VN, Hatfield DL (2008) A functional link between housekeeping selenoproteins and phase II enzymes. Biochem J 413:151–161. https://doi.org/10.1042/bj20080277
Schomburg L, Schweizer U (2009) Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim Biophys Acta 1790:1453–1462. https://doi.org/10.1016/j.bbagen.2009.03.015
Schweizer U, Fradejas-Villar N (2016) Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J 30:3669–3681. https://doi.org/10.1096/fj.201600424
Howard MT, Carlson BA, Anderson CB, Hatfield DL (2013) Translational redefinition of UGA codons is regulated by selenium availability. J Biol Chem 288:19401–19413. https://doi.org/10.1074/jbc.M113.481051
Donovan J, Copeland PR (2010) The efficiency of selenocysteine incorporation is regulated by translation initiation factors. J Mol Biol 400:659–664. https://doi.org/10.1016/j.jmb.2010.05.026
Riese C, Michaelis M, Mentrup B, Gotz F, Kohrle J, Schweizer U, Schomburg L (2006) Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology 147:5883–5892. https://doi.org/10.1210/en.2006-0689
Becker NP, Martitz J, Renko K, Stoedter M, Hybsier S, Cramer T, Schomburg L (2014) Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics 6:1079–1086. https://doi.org/10.1039/c4mt00004h
Stoedter M, Renko K, Hog A, Schomburg L (2010) Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J 429:43–51. https://doi.org/10.1042/Bj20091868
Moosmann B, Behl C (2004) Selenoproteins, cholesterol-lowering drugs, and the consequences: revisiting of the mevalonate pathway. Trends Cardiovasc Med 14:273–281. https://doi.org/10.1016/j.tcm.2004.08.003
Renko K, Martitz J, Hybsier S, Heynisch B, Voss L, Everley RA, Gygi SP, Stoedter M et al (2017) Aminoglycoside-driven biosynthesis of selenium-deficient selenoprotein P. Sci Rep-Uk 7. https://doi.org/10.1038/s41598-017-04586-9 ARTN 4391
Mariotti M, Shetty S, Baird L, Wu S, Loughran G, Copeland PR, Atkins JF, Howard MT (2017) Multiple RNA structures affect translation initiation and UGA redefinition efficiency during synthesis of selenoprotein P. Nucleic Acids Res 45. https://doi.org/10.1093/nar/gkx982
Berry MJ, Maia AL, Kieffer JD, Harney JW, Larsen PR (1992) Substitution of cysteine for selenocysteine in type-i iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology 131:1848–1852. https://doi.org/10.1210/en.131.4.1848
Schomburg L, Schweizer U, Holtmann B, Flohe L, Sendtner M, Kohrle J (2003) Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J 370:397–402. https://doi.org/10.1042/Bj20021853
Hill KE, Zhou J, Mcmahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF (2003) Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 278:13640–13646. https://doi.org/10.1074/jbc.M300755200
Hill KE, Zhou J, Mcmahan WJ, Motley AK, Burk RF (2004) Neurological dysfunction occurs in mice with targeted deletion of the selenoprotein P gene. J Nutr 134:157–161. https://doi.org/10.1093/jn/134.1.157
Renko K, Werner M, Renner-Muller I, Cooper TG, Yeung CH, Hollenbach B, Scharpf M, Kohrle J et al (2008) Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem J 409:741–749. https://doi.org/10.1042/BJ20071172
Beck MA (1999) Selenium and host defence towards viruses. Proc Nutr Soc 58:707–711. https://doi.org/10.1017/S0029665199000920
Beck MA, Shi Q, Morris VC, Levander OA (1995) Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med 1:433–436
Yu FF, Han J, Wang X, Fang H, Liu H, Guo X (2016) Salt-rich selenium for prevention and control children with Kashin-Beck disease: a meta-analysis of community-based trial. Biol Trace Elem Res 170:25–32. https://doi.org/10.1007/s12011-015-0437-x
Zhou H, Wang T, Li Q, Li D (2018) Prevention of Keshan disease by selenium supplementation: a systematic review and meta-analysis. Biol Trace Elem Res 186:98–105. https://doi.org/10.1007/s12011-018-1302-5
Broome CS, Mcardle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80:154–162. https://doi.org/10.1093/ajcn/80.1.154
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients 10. https://doi.org/10.3390/nu10091203
Wu Q, Rayman MP, Lv HJ, Schomburg L, Cui B, Gao CQ, Chen P, Zhuang GH et al (2015) Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab 100:4037–4047. https://doi.org/10.1210/jc.2015-2222
Wang Y, Zhao FY, Rijntjes E, Wu LP, Wu Q, Sui J, Liu YF, Zhang M et al (2019) Role of selenium intake for risk and development of hyperthyroidism. J Clin Endocrinol Metab 104:568–580. https://doi.org/10.1210/jc.2018-01713
Schomburg L, Riese C, Michaelis M, Griebert E, Klein MO, Sapin R, Schweizer U, Kohrle JK (2006) Synthesis and metabolism of thyroid hormones is preferentially maintained in selenium-deficient transgenic mice. Endocrinology 147:1306–1313. https://doi.org/10.1210/en.2005-1089
Chiu-Ugalde J, Wirth EK, Klein MO, Sapin R, Fradejas-Villar N, Renko K, Schomburg L, Kohrle J et al (2012) Thyroid function is maintained despite increased oxidative stress in mice lacking selenoprotein biosynthesis in thyroid epithelial cells. Antioxid Redox Signal 17:902–913. https://doi.org/10.1089/ars.2011.4055
Duntas LH (2010) Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab 95:5180–5188. https://doi.org/10.1210/jc.2010-0191
Effraimidis G, Wiersinga WM (2014) Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol 170:R241–R252. https://doi.org/10.1530/eje-14-0047
Wichman J, Winther KH, Bonnema SJ, Hegedus L (2016) Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid 26:1681–1692. https://doi.org/10.1089/thy.2016.0256
Schomburg L (2012) Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 8:160–171. https://doi.org/10.1038/nrendo.2011.174
Rayman MP (2012) Selenium and human health. Lancet (London, England) 379:1256–1268. https://doi.org/10.1016/s0140-6736(11)61452-9
Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52:812–817. https://doi.org/10.2337/diabetes.52.3.812
Hughes DJ, Duarte-Salles T, Hybsier S, Trichopoulou A, Stepien M, Aleksandrova K, Overvad K, Tjonneland A et al (2016) Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 104:406–414. https://doi.org/10.3945/ajcn.116.131672
Hughes DJ, Fedirko V, Jenab M, Schomburg L, Meplan C, Freisling H, Bueno-De-Mesquita HB, Hybsier S et al (2015) Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer 136:1149–1161. https://doi.org/10.1002/ijc.29071
Schomburg L, Hughes DJ (2017) The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev Gastroent 11:707–709. https://doi.org/10.1080/17474124.2017.1320219
Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A et al (2007) Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 35:118–126. https://doi.org/10.1097/01.ccm.0000251124.83436.0e
Forceville X, Vitoux D, Gauzit R, Combes A, Lahilaire P, Chappuis P (1998) Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med 26:1536–1544
Braunstein M, Kusmenkov T, Zuck C, Angstwurm M, Becker NP, Bocker W, Schomburg L, Bogner-Flatz V (2019) Selenium and selenoprotein P deficiency correlates with complications and adverse outcome after major trauma. Shock. https://doi.org/10.1097/shk.0000000000001344
Rayman MP, Bath SC, Westaway J, Williams P, Mao J, Vanderlelie JJ, Perkins AV, Redman CW (2015) Selenium status in U.K. pregnant women and its relationship with hypertensive conditions of pregnancy. Br J Nutr 113:249–258. https://doi.org/10.1017/s000711451400364x
Bizerea TO, Dezsi SG, Marginean O, Stroescu R, Rogobete A, Bizerea-Spiridon O, Ilie C (2018) The link between selenium, oxidative stress and pregnancy induced hypertensive disorders. Clin Lab 64:1593–1610. https://doi.org/10.7754/Clin.Lab.2018.180307
Schomburg L, Orho-Melander M, Struck J, Bergmann A, Melander O (2019) Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients 11. https://doi.org/10.3390/nu11081852
Castro Aguilar-Tablada T, Navarro-Alarcon M, Quesada Granados J, Samaniego Sanchez C, Rufian-Henares JA, Nogueras-Lopez F (2016) Ulcerative colitis and Crohn’s disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients 8. https://doi.org/10.3390/nu8120780
Alehagen U, Johansson P, Bjornstedt M, Rosen A, Post C, Aaseth J (2016) Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur J Clin Nutr 70:91–96. https://doi.org/10.1038/ejcn.2015.92
Koszta G, Kacska Z, Szatmari K, Szerafin T, Fulesdi B (2012) Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac surgery. J Anesth 26:812–821. https://doi.org/10.1007/s00540-012-1454-y
Jujic A, Melander O, Bergmann A, Hartmann O, Nilsson PM, Bachus E, Struck J, Magnusson M (2019) Selenoprotein P deficiency and risk of mortality and rehospitalization in acute heart failure. J Am Coll Cardiol 74:1009–1011. https://doi.org/10.1016/j.jacc.2019.06.023
Mccann JC, Ames BN (2011) Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J 25:1793–1814. https://doi.org/10.1096/fj.11-180885
Wang YB, Gao X, Pedram P, Shahidi M, Du JL, Yi YQ, Gulliver W, Zhang HW et al (2016) Significant beneficial association of high dietary selenium intake with reduced body fat in the CODING Study. Nutrients 8. https://doi.org/10.3390/nu8010024 ARTN 24
Ortega RM, Rodriguez-Rodriguez E, Aparicio A, Jimenez-Ortega AI, Palmeros C, Perea JM, Navia B, Lopez-Sobaler AM (2012) Young children with excess of weight show an impaired selenium status. Int J Vitam Nutr Res 82:121–129. https://doi.org/10.1024/0300-9831/a000101
Schomburg L (2016) Dietary selenium and human health. Nutrients 9. https://doi.org/10.3390/nu9010022
Vinceti M, Maraldi T, Bergomi M, Malagoli C (2009) Risk of chronic low-dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health 24:231–248
Plows JF, Reynolds CM, Vickers MH, Baker PN, Stanley JL (2019) Nutritional supplementation for the prevention and/or treatment of gestational diabetes mellitus. Curr Diab Rep 19:73. https://doi.org/10.1007/s11892-019-1199-1
Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A et al (2007) Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 147:217–223. https://doi.org/10.7326/0003-4819-147-4-200708210-00175
Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB et al (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6:361–369. https://doi.org/10.1016/S2213-8587(18)30051-2
Kohler LN, Foote J, Kelley CP, Florea A, Shelly C, Chow HS, Hsu P, Batai K et al (2018) Selenium and type 2 diabetes: systematic review. Nutrients 10. https://doi.org/10.3390/nu10121924
Kohler LN, Florea A, Kelley CP, Chow S, Hsu P, Batai K, Saboda K, Lance P et al (2018) Higher plasma selenium concentrations are associated with increased odds of prevalent type 2 diabetes. J Nutr 148:1333–1340. https://doi.org/10.1093/jn/nxy099
Mao JY, Teng WP (2013) The relationship between selenoprotein P and glucose metabolism in experimental studies. Nutrients 5:1937–1948. https://doi.org/10.3390/nu5061937
Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H (2008) Selenoprotein P expression is controlled through interaction of the coactivator PGC-1 alpha with FoxO1a and hepatocyte nuclear factor 4 alpha transcription factors. Hepatology 48:1998–2006. https://doi.org/10.1002/hep.22526
Speckmann B, Sies H, Steinbrenner H (2009) Attenuation of hepatic expression and secretion of selenoprotein P by metformin. Biochem Bioph Res Co 387:158–163. https://doi.org/10.1016/j.bbrc.2009.06.143
Jin Y, Chung YW, Jung MK, Lee JH, Ko KY, Jang JK, Ham M, Kang H et al (2019) Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes. Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03287-y
Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC (2002) Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomark Prev 11:630–639
Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG et al (2011) Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306:1549–1556. https://doi.org/10.1001/jama.2011.1437
Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, Broadley MR, Motley AK et al (2010) Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 91:923–931. https://doi.org/10.3945/ajcn.2009.28169
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM et al (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301:39–51. https://doi.org/10.1001/jama.2008.864
Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH et al (2016) Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst 108. https://doi.org/10.1093/jnci/djw152
Jacobs ET, Lance P, Mandarino LJ, Ellis NA, Chow HS, Foote J, Martinez JA, Hsu CP et al (2019) Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Res Care 7:e000613. https://doi.org/10.1136/bmjdrc-2018-000613
Morris JS, Crane SB (2013) Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 5:1024–1057. https://doi.org/10.3390/nu5041024
D’oria L, Apicella M, De Luca C, Licameli A, Neri C, Pellegrino M, Simeone D, De Santis M (2018) Chronic exposure to high doses of selenium in the first trimester of pregnancy: case report and brief literature review. Birth Defects Res 110:372–375. https://doi.org/10.1002/bdr2.1148
Nuttall KL (2006) Evaluating selenium poisoning. Ann Clin Lab Sci 36:409–420
Varo P, Alfthan G, Ekholm P, Aro A, Koivistoinen P (1988) Selenium intake and serum selenium in Finland: effects of soil fertilization with selenium. Am J Clin Nutr 48:324–329. https://doi.org/10.1093/ajcn/48.2.324
Brodin O, Eksborg S, Wallenberg M, Asker-Hagelberg C, Larsen EH, Mohlkert D, Lenneby-Helleday C, Jacobsson H et al (2015) Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: the SECAR Study. Nutrients 7:4978–4994. https://doi.org/10.3390/nu7064978
Acknowledgments
Research in the LS lab is conducted by a number of dedicated, diligent and excellent co-workers, technicians and students, and financially supported by the Deutsche Forschungsgemeinschaft (DFG Research Unit 2558 TraceAge, Scho 849/6-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LS holds shares in selenOmed GmbH, a company involved in Se status assessment and supplementation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schomburg, L. The other view: the trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 19, 15–24 (2020). https://doi.org/10.1007/s42000-019-00150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-019-00150-4