Abstract
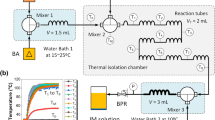
The Pinnick oxidation, due to its tolerance for sensitive functional groups, is widely used in the process of oxidizing α,β-unsaturated aldehydes to corresponding carboxylic acids. The reaction reagents typically include sodium chlorite, buffer salts, and a scavenger. However, the controllability of Pinnick oxidation in the batch reaction process is poor due to the inherent limitations of the reactor’s performance. This leads to potential safety risks and necessitates the reaction to proceed slowly under conditions of low temperature and low concentration. In this work, we introduced a new continuous micro-reaction process to intensify the Pinnick oxidation. The water-soluble crotonic acid was selected as a typical object of study. Through the study of reaction parameters and the construction of a micro-reaction system, efficient continuous process was achieved under high-temperature and high-pressure conditions for the first time. Compared to the batch process, the reaction benefited from the superheated condition resulting in a significant acceleration of the reaction rate, efficient gas–liquid interphase mass transfer allowing for effective utilization of the generated chlorine dioxide, and the inherent safety of the microreactor enabling an increase in reaction concentration. In addition, the buffer salts used in the Pinnick oxidation has been successfully replaced by hydrochloric acid and applied to the continuous flow. This work shows the tremendous potential of microreactors in utilizing harsh reaction conditions to achieve process intensification.
Graphical abstract

Article Highlights
-
A superheated micro-reaction process was introduced into the Pinnick oxidation to achieve efficient and safe preparation of crotonic acid.
-
Efficient gas–liquid interphase mass transfer in microreactor realized effective utilization of the by-product chlorine dioxide.
-
The great replacement of phosphate buffer salts with hydrochloric acid was achieved in continuous flow.












Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Krapcho AP (2006) Org Prep Proced Int 38:177–216. https://doi.org/10.1080/00304940609355988
Bal BS, Childers WE, Pinnick HW (1981) Tetrahedron 37:2091–2096. https://doi.org/10.1016/S0040-4020(01)97963-3
Lindgren BO, Nilsson T (1973) Acta Chem Scand 27:888–890. https://doi.org/10.3891/acta.chem.scand.27-0888
Mannam S, Sekar G (2008) Tetrahedron Lett 49:1083–1086. https://doi.org/10.1016/j.tetlet.2007.11.198
Kon Y, Imao D, Nakashima T, Sato K (2009) Chem Lett 38:430–431. https://doi.org/10.1246/cl.2009.430
Mahmood A, Robinson GE, Powell L (1999) Org Process Res Dev 3:363–364. https://doi.org/10.1021/op990021h
Hunsen M (2005) Synthesis 2005:2487–2490. https://doi.org/10.1055/s-2005-872085
Bowden K, Heilbron IM, Jones ERH, Weedon BCL (1946) J Chem Soc (Resumed): 39–45. https://doi.org/10.1039/JR9460000039
Travis BR, Sivakumar M, Hollist GO, Borhan B (2003) Org Lett 5:1031–1034. https://doi.org/10.1021/ol0340078
Baumeister T, Kitzler H, Obermaier K, Zikeli S, Röder T (2015) Org Process Res Dev 19:1576–1579. https://doi.org/10.1021/acs.oprd.5b00173
Liu K-J, Fu Y-L, Xie L-Y, Wu C, He W-B, Peng S, Wang Z, Bao W-H, Cao Z, Xu X, He W-M (2018) ACS Sustain Chem Eng 6:4916–4921. https://doi.org/10.1021/acssuschemeng.7b04400
Dai PF, Qu JP, Kang YB (2019) Org Lett 21:1393–1396. https://doi.org/10.1021/acs.orglett.9b00101
Tanaka S, Kon Y, Uesaka Y, Morioka R, Tamura M, Sato K (2016) Chem Lett 45:188–190. https://doi.org/10.1246/cl.151024
Fedevich OE, Levush SS, Fedevich EV, Kit YV (2003) Russ J Org Chem 39:29–32. https://doi.org/10.1023/A:1023482226774
Vanoye L, Abdelaal M, Grundhauser K, Guicheret B, Fongarland P, De Bellefon C, Favre-Reguillon A (2019) Org Lett 21:10134–10138. https://doi.org/10.1021/acs.orglett.9b04193
Vanoye L, Favre-Réguillon A (2022) Org Process Res Dev 26:335–346. https://doi.org/10.1021/acs.oprd.1c00399
Lehtinen C, Brunow G (2000) Org Process Res Dev 4:544–549. https://doi.org/10.1021/op000045k
Murakami K, Toma T, Fukuyama T, Yokoshima S (2020) Angew Chem Int Ed 59:6253–6257. https://doi.org/10.1002/anie.201916611
Ishihara J, Hagihara K, Chiba H, Ito K, Yanagisawa Y, Totani K (2000) Tadano K-i. Tetrahedron Lett 41:1771–1774. https://doi.org/10.1016/S0040-4039(00)00013-7
Kuramochi K, Nagata S, Itaya H (1999) Takao K-i, Kobayashi S. Tetrahedron Lett 40:7371–7374. https://doi.org/10.1016/S0040-4039(99)01512-9
Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K (2004) Science 305:495–499. https://doi.org/10.1126/science.1098851
Nicolaou KC, Edmonds DJ, Li A, Tria GS (2007) Angew Chem Int Ed 46:3942–3945. https://doi.org/10.1002/anie.200700586
Magauer T, Martin HJ, Mulzer J (2009) Angew Chem Int Ed 48:6032–6036. https://doi.org/10.1002/anie.200900522
Hussein AA, Al-Hadedi AAM, Mahrath AJ, Moustafa GAI, Almalki FA, Alqahtani A, Shityakov S, Algazally ME (2020) R Soc Open Sci 7:191568. https://doi.org/10.1098/rsos.191568
Dalcanale E, Montanari F (1986) J Org Chem 51:567–569. https://doi.org/10.1021/jo00354a037
Raach A, Reiser O (2000) J Prakt Chem 342:605–608. https://doi.org/10.1002/1521-3897(200006)342:6%3c605::Aid-prac605%3e3.3.Co;2-9
Hessel V, Kralisch D, Kockmann N, Noel T, Wang Q (2013) Chemsuschem 6:746–789. https://doi.org/10.1002/cssc.201200766
Hessel V, Cortese B, De Croon M (2011) Chem Eng Sci 66:1426–1448. https://doi.org/10.1016/j.ces.2010.08.018
Jensen KF, Reizman BJ, Newman SG (2014) Lab Chip 14:3206–3212. https://doi.org/10.1039/c4lc00330f
Jensen KF (2017) AIChE J 63:858–869. https://doi.org/10.1002/aic.15642
Nagao I, Ishizaka T, Kawanami H (2016) Green Chem 18:3494–3498. https://doi.org/10.1039/c6gc01195k
Razzaq T, Kappe CO (2010) Chem Asian J 5:1274–1289. https://doi.org/10.1002/asia.201000010
Shang M, Noël T, Su Y, Hessel V (2016) Ind Eng Chem Res 55:2669–2676. https://doi.org/10.1021/acs.iecr.5b04813
Huang J-P, Sang F-N, Luo G-S, Xu J-H (2017) Chem Eng Sci 173:507–513. https://doi.org/10.1016/j.ces.2017.08.020
Huang J, Geng Y, Wang Y, Xu J (2019) Ind Eng Chem Res 58:16389–16394. https://doi.org/10.1021/acs.iecr.9b02438
Phung Hai TA, Samoylov AA, Rajput BS, Burkart MD (2022) ACS Omega 7:15350–15358. https://doi.org/10.1021/acsomega.1c06823
Jin RY, Hu SQ, Zhang YH, Bo T (2008) Chin Chem Lett 19:1375–1378. https://doi.org/10.1016/j.cclet.2008.09.001
Boucher MM, Furigay MH, Quach PK, Brindle CS (2017) Org Process Res Dev 21:1394–1403. https://doi.org/10.1021/acs.oprd.7b00231
Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of
The supplementary China (22108264, 22378376) for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Li, Y., Zhou, Y. et al. Efficient pinnick oxidation by a superheated micro-reaction process. J Flow Chem (2024). https://doi.org/10.1007/s41981-024-00324-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41981-024-00324-1