Abstract
The number of biocatalyzed reactions at industrial level is growing rapidly together with our understanding on how we can maximize the enzyme efficiency, stability and productivity. While biocatalysis is nowadays recognized as a greener way to operate in chemistry, its combination with continuous processes has lately come up as a powerful tool to enhance process selectivity, productivity and sustainability. This perspective aims at describing the recent advances of this technology and future developments leading to smart, efficient and greener strategies for process optimization and large-scale production.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ages ago our ancestors started to prepare food and drinks via yeast cells, thus applying for the first time enzyme technology to high-value products, although without consciousness. The addition of yeast containing its own enzymes made possible the fermentation for bread preparation as well as the transformation of glucose in ethanol during brewing [1]. Just in the middle 1800s the first enzymes were discovered (i.e., a mixture of amylases), and since then, enzyme-mediated processes have been introduced in several sectors starting from food manufacturing. More recently, one or more biocatalytic steps have been added to the preparation of fine chemicals such as pharmaceuticals, cosmetics and agrochemicals [2]. Among the most impressive examples in the pharmaceutical field the synthesis of Islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI) for HIV infection, which has been achieved exclusively enzymatically in 2019 by Merck [3] as well as the heavily engineered transaminase ATA 117, key catalyst for the preparation of the blockbuster drug Sitagliptin [4] are noteworthy. Additionally, a protein from tropical berries, prepared by Amai Proteins company, being 3000 times sweeter than sugar, seems to possess the potential to replace up to 70% of the added sugars without health hazards and off-flavors of the synthetic and natural sweeteners. While its stability was enhanced via protein engineering, its production on large scale was carried out through yeast technology [5]. What is particularly clear is that protein production is no longer a problem, and the concern about biocatalysis as an expensive niche technology has been dismantled. Particularly, with the progress of enzyme engineering, which allowed for the inclusion of Prof. Frances Arnold among the Nobel laureates, precise design of enzymes became possible, making biocatalysis an efficient tool compatible with industrial needs. In a parallel way, a particular focus has been put in carrying out highly-performing reactions, shifting the technical set-up from batch mode to alternative solutions. Among them, catalyst stabilization via enzyme immobilization techniques and flow chemistry technology, especially when combined, represent a leap forward in enhancing the sustainability, safety and productivity of biotransformations, thus impacting on the process efficiency and process-related costs [6,7,8]. Very recently, multi-enzymatic flow bioreactions merging the advantages of flow facilities (i.e., better parameter control, higher mass and heat transfer, modularity), with the mild reaction conditions and the selectivity of biocatalysts (i.e., water media, room temperature, chemo-, regio-, and stereoselectivity) were even able to efficiently mimic cell metabolic pathways [9, 10].
Sustainable biocatalysis
Biocatalysis, which has been promoted as a cheaper, cleaner and more environmentally-friendly technology compared to conventional chemistry, leads to a rapid growth in the number of scientific publications studying its sustainability and increasing reports of biocatalyzed processes running on a commercial scale [11].
Sustainability in biocatalysis involves several key principles that collectively contribute to the overall goal of minimizing environmental impact, conserving resources, and promoting catalyst long-term viability. Firstly, enzymatic processes offer several advantages in this regard due to their ability to operate under mild conditions. Unlike traditional chemical methods that often require harsh temperatures and toxic chemicals, biocatalysis can proceed at physiologically-like conditions, which significantly reduce energy consumption and the generation of hazardous waste.
Furthermore, sustainable biocatalysis emphasizes resource efficiency thanks to high substrate specificity, meaning that enzymes can catalyze target reactions with minimal waste production and formation of undesired by-products. Additionally, the recycling of enzymes, co-factors and solvents further optimize resource utilization, minimizing the requirement for fresh inputs [12].
Sustainable biocatalysis also aligns with circular economy principles. By recycling waste streams or residues from other processes as substrates, bioreactions can transform discarded materials into valuable products. This approach enhances resource utilization and supports a more circular and self-sustaining strategy for high value molecule production. While addressing environmental and ethical concerns is a key factor in the circular economy context, a sustainable biocatalytic process must also take into consideration the economic viability by evaluating process cost-effectiveness, optimizing catalyst performance, and minimizing production expenses. The development of efficient and scalable strategies that yield valuable products contributes to the overall financial feasibility of a biocatalytic process. A comprehensive evaluation of sustainability involves lifecycle assessment (LCA), a standardized and internationally recognized tool (ISO 14044:2006) which examines the entire lifecycle of a product or process from its inception to its disposal. This approach helps identifying areas of potential environmental impact and guides efforts aimed at mitigating these effects. Enzymatic synthesis typically exhibits a lower environmental impact compared to chemical one. This is primarily attributed to reduced reaction times (correlated with energy consumption), enhanced substrate selectivity and increased isolated yield. Notably, the recycling of solvents and enzymes as well as the use of continuous automated strategies emerge as the most impactful parameters [13]. In the last decade several works comparing LCAs between biocatalytic/chemical approaches identified and quantified the disparities between the different two processes, thus highlighting the importance of making this evaluation a commonly-used procedure [14, 15].
Overall, sustainability in biocatalysis includes a forward-looking and responsible approach to chemical synthesis. By integrating environmental management, resource efficiency, circular economy principles, economic viability, and social responsibility, sustainable biocatalysis aims at combining technological advancements with the preservation of safe environments.
How to measure the sustainability of a chemical process
In recent decades, biocatalytic processes have induced a significant transformation within the landscape of chemical synthesis. These methodologies have not only emerged as valuable complements to traditional chemical synthesis but have, in certain cases, demonstrated their potential as viable substitutes. This shift is fueled by the imperative to harness renewable and cost-effective raw materials, embrace environmentally sustainable synthetic routes, minimize the generation of hazardous by-products and waste, while ensuring high quality and value of the end product. However, the correlation between mild conditions and the overall system sustainability necessitates rigorous evaluation, particularly during the early stages of process development.
Being biocatalysis a young technology compared with established conventional chemical catalysis, evaluating the environmental footprint of bioprocesses is often complex due to limited available data [16, 17]. Nevertheless, different "Green Chemistry Metrics", have been introduced to quantitatively assess the environmental performance of processes.
Atom efficiency (AE) and carbon mass efficiency (CME) are two pivotal metrics which specifically focused on evaluating the reaction chemistry. AE, also known as atom economy, measures the proportion of starting materials that contribute to the desired end product (Table 1, entry 1). Based on the reaction stoichiometry and mechanism, it helps to design reactions that integrate a maximum number of substrate atoms into the final product structure [18]. In contrast, CME assesses the percentage of carbon in the reagents that contributes to the final product (Table 1, entry 2) [19]. The central goal of evaluating AE and CME values during the early stage of the process is to establish benchmarks for its development and implementation. However, these metrics evaluate the chemistry eco-friendliness without considering the whole process (e.g., by-products, co-substrates, and solvents).
The E-factor assesses the waste generated during the synthesis of chemical compounds, quantifying it as the amount of waste produced per kilogram of product (Table 1, entry 3) [12]. In the E-factor calculation, waste includes everything that leaves the process boundaries except for the desired product [20]. While water, considered an environmentally-friendly solvent, is typically excluded from the E-factor calculation, many chemical processes require highly-purified water, whose obtainment and disposal present its own ecological impact [21]. Consequently, two variants of the E-factor are available: one excluding water and another one including this solvent [22]. The major limitation of E-factor metric is that it does not account for the specific type of by-products or waste generated.
Reaction mass efficiency (RME), also known as mass efficiency, is an updated version of the E-factor. It takes into consideration the yield of the reaction, the actual amounts of reagents used, and the atom economy. To calculate RME, the mass of the product is divided by the total mass of all the reagents involved in the process (Table 1, entry 5) [18].
The Effective Mass Yield (EMY) is a metric designed to provide insights into the overall efficiency of a chemical process in terms of mass utilization. EMY quantifies the portion of the total mass of reactants that effectively contributes to the formation of the desired product in correlation with all the non-benign materials used in its synthesis (Table 1, entry 5) [23]. This metric helps highlight how efficiently the reactants are being utilized to yield the final product, even if it fails to define non-benign reagents with precision.
Process mass intensity (PMI) is a metric embraced by the American Chemical Society Green Chemistry Institute's Pharmaceutical Roundtable as a high-level measure to assess the sustainability of a manufacturing process [24]. PMI is defined as the total mass of materials required to produce a specified mass of the end product (Table 1, entry 6). Additionally, PMI incorporates catalyst production and downstream processes needed for isolating and purifying the final product. However, PMI does not include specific concerns related to the environmental impact, health, and safety of raw materials or waste produced [25].
Regarding the utilization of solvents, alternative metrics have been proposed to effectively assess their impact on the overall environmental performance of a process. One such metric is Solvent Intensity (SI), developed to address the limitations of the E-factor when evaluating solvent usage. SI aims at analyzing and quantifying the total amount of all solvents employed within the whole process (Table 1, entry 7) [20]. A specific variant of SI is Water Intensity (WI), which focuses on evaluating the quantity of water, (Table 1, entry 8) [26] holds particular significance in the context of biocatalysis and fermentation processes, where one of the key advantages is the potential to operate with environmentally benign solvents, primarily water (Table 1, entry 8) [26]. This is especially noteworthy considering that water frequently constitutes a significant portion, often over 50%, of the total resource mass. However, it is crucial to acknowledge that water has transformed into a limited and overexploited natural resource, prompting the need for responsible management [27, 28].
The evaluation of all these metrics, together with many other proposed, contribute to a comprehensive vision of the greenness and efficiency of a chemical process. By incorporating these metrics into process assessments, researchers and industries can make informed decisions to optimize resource utilization, minimize waste generation, and advance sustainable practices in chemical synthesis.
How biocatalysis flows
Enzyme Immobilization
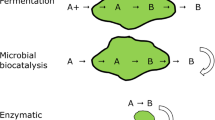
Although both free and immobilized biocatalysts have been employed under flow conditions, the immobilized ones are more commonly used, thus generating a heterogeneous system where the enzymes retain sufficient structure flexibility for the reaction to take place [8, 29, 30]. Various procedures regarding enzyme immobilization of whole-cell biocatalysts or purified enzymes have been reported, adopting a variety of different carriers. Both the strategies present pros and cons. Since our research group favors cell-free enzymes as biocatalysts (Fig. 1), we decided to focus on this topic suggesting the reading of the review by Pinto et al. for the most recent advances on whole cell immobilization [31]. Unlike whole cell systems, the use of pure enzymes avoids permeability issues due to the absence of cell membrane/cell wall as well as reduced overall final yields because of competing cell secondary pathways. Moreover, it allows for the employment of a specific amount of a catalyst (i.e., catalyst loading) so that, in a multi-step enzymatic cascade, each reaction can be individually fine-tuned finally obtaining a fully optimized process [9, 32]. It has been already demonstrated that enzyme immobilization impacts on the catalyst stability; this is particularly true for covalent immobilization strategies [33, 34].
Most popular pure enzyme immobilization techniques, adapted from Pinto et al. [31]
In our group we have developed a range of different catalyst immobilization based on covalent bonds between the protein and the matrix to finally obtain robust and durable biocatalysts to be specifically used under flow conditions, for high-productivity processes. Among the most successful procedures the one involving the interaction between the enzymatic poly-His-tag, typically fused with the desired protein during the cloning phase to allow for its purification, together with bivalent metal derivatized-epoxy-supports, are noteworthy [35, 36]. The convenience of this protocol stands in the fact that the carriers are commercially available (e.g., various pore size, different linkers etc.), while the tunability is outstanding since the metal involved in the coordination with the protein His-tag (Fig. 2a) can be selected to avoid catalyst poisoning and its consequent inactivation. Furthermore, the enzyme-matrix contact time can be varied to improve/decrease the covalent bond formation, thus impacting on the final catalyst stability (e.g., rigidification/distortion of the protein structure). Moreover, since the addition of the poly-His-tag is routinely employed to obtain a pure protein via metal affinity chromatography, cell crude extracts can be used as starting point for the immobilization procedure, thus combining in one-step protein purification and its immobilization [32].
Although a number of different hydrophobic and hydrophilic carriers with different bead diameters, pore size and various linkers are available, non-swelling resins should be preferred for flow processing, especially when segmented streams are involved (e.g., aqueous/organic solvents or gas/liquid phases). In this way back pressure is minimized and no change in the volume is observed.
However, neither the matrix nor the chemistry is universally ideal, and for every biocatalyst a trial-and-error procedure should be used to finally identify the best immobilization strategy. As an example, the acyl transferase from Mycobacterium smegmatis (MsAcT) has been immobilized on a series of different supports, but the best retained activity and immobilization yield was achieved using the hydrophilic agarose previously functionalized with aldehyde groups (i.e., glyoxyl-agarose) [34]. Employing this method, a covalent bond was generated between the superficial lysine residues of the protein and the aldehyde groups (i.e., imine formation) which was subsequently reduced via NaBH4, thus forming the stable amine bond (Fig. 2b).
Although during the last 20 years multi-step biotransformations have been performed by separately immobilized enzymes [9, 37], co-immobilization of several proteins on the same carrier demonstrated to enhance the catalytic performance by:
-
(1)
reducing reaction steps,
-
(2)
minimizing by-product formation,
-
(3)
decreasing the accumulation of unstable/toxic intermediates,
-
(4)
shifting the thermodynamic equilibrium toward the target product,
-
(5)
recycling enzyme cofactors in situ (when necessary),
-
(6)
increasing bioprocess productivity and cost-efficiency [38, 39].
As an example, a commercially available α-rhamnosidase (RN) and an extremophilic home-made β-glycosidase (HOR) have been co-immobilized on glyoxyl-agarose beads to prepare a high-performing multi-active biocatalyst (imm-RN-HOR) for the “one-shot” obtainment of aglycones starting from natural rutinosides (i.e., glycosides containing rhamnose and glucose moieties)[40].
A significant amount of research focuses on the optimization of immobilization protocols, especially in the preparation of a searchable database to guide the selection of the best immobilization method for a specific biocatalyst avoiding the cost-, time- and energy-consuming trial-and-error procedure [41]. Another key point regards the sustainability of the support preparation before enzyme immobilization, which is usually carried out via conventional chemical methods employing toxic or hazardous reagents (e.g., strong acids, oxidants etc.). Our research group is focusing now on the development of biocatalyzed procedures for the support functionalization as well as the employment of natural polymers (better if recovered from waste, residues or by-products) as carrier for enzyme immobilization. On one side we aim at enhancing the greenness of these processes, on the other side we would like to increase the use of immobilized enzymes in particular sectors where the consumer preference for compounds derived from natural sources is rapidly expanding (e.g., food, cosmetic fields).
Solvents in biocatalysis
Solvents are widely used in various industrial and chemical sectors and, despite high precautions, they inevitably lead to contamination of air, land and water due to their volatile nature and difficulty in containment. Scientific literature offers a range of solvent selection guides provided by entities such as GSK, Pfizer, AstraZeneca, and the American Chemical Society (ACS) [24]. These guidelines prioritizing safety, health, and environmental concerns, aim to responsible and greener solvent choices.
Upon an in-depth examination of various chemical processes, it becomes evident that solvents play a key role as significant contributors to overall mass and performance enhancement [26]. Their strategic use often results in improved yields, thus enhancing the environmental efficacy of overall processes. This phenomenon is particularly evident in the fine chemicals and pharmaceutical production sectors, where solvents typically account for 75% of total energy consumption, approximately 70% of volatile organic compounds (VOCs) emissions, and around 50% of the global warming potential (GWP) [42].
Since most organic solvents are still derived from petroleum-based sources, the production process itself significantly contributes to their overall environmental pollution [43, 44].
Even if organic solvents are generally classified as non-sustainable, there is a significant variability in environmental impact between different solvents, thus considerations about environmental implications, safety risks, and handling requirements are essential when choosing a solvent for process development. Those one classified as highly-risky should ideally be avoided or limited as much as possible. Other aspects to be taken into account involve ease of separation, product recovery, and catalyst compatibility, as well as possibility of solvent recycling and reuse. In the last years, many industries have made remarkable progress in implementing “closed-loop systems” that reduce solvent and/or water employment and improve their recycle and reuse [27]. For instance, water-miscible solvents commonly undergo recovery through distillation and evaporation, while immiscible solvents are often recovered using liquid–liquid extraction [45].
Water, as previously mentioned, is traditionally the solvent of choice for biocatalytic reactions, offering a big advantage for the overall process sustainability. However, bioprocesses typically generate large volumes of wastewater, necessitating proper treatments that tend to be energy-intensive [46]. This aspect highlights the importance of evaluating not only the solvent itself but also the downstream effects and environmental implications associated with its usage. While water remains an attractive option for its alignment with green chemistry principles, its sustainable utilization requires a comprehensive evaluation that extends beyond its immediate use, encompassing considerations of scarcity, waste generation, and energy consumption.
Over the last 15 years, there has been an increasing interest from both academic and industrial sectors towards unconventional solvent alternatives. Among these, ionic liquids (IL), supercritical fluids (SCF) and fluorous solvents present significant environmental benefits over conventional organic solvents.
ILs aquired popularity due to several advantages, including high thermal stability, low vapor pressure, and adjustable viscosity and miscibility in aqueous environments [47]. In addition, their non-volatile nature allows for an easy and full recovery [48]. Nevertheless, ongoing evaluations are addressing concerns about potential toxicity [48, 49].
Fluorous solvents, a term coined by Horvath, refer to highly fluorinated alkanes, ethers and tertiary amines that are suitable for biphasic catalysis due to their temperature-dependent miscibility with conventional organic solvents [50]. Regarding their application in biocatalysis, the main limitation seems to be the high cost and the low solubility of enzymes in these solvents [51].
Lastly, supercritical fluids are non-flammable solvents which present properties between liquids and gases, since their density is comparable to a liquid, whereas their viscosity is similar to that of gases [52]. sc-CO2 is the most widely used for biocatalyzed processes because it is cheap, chemically inert and readily available [53].
In this area, an emerging working line is the use of bio-solvents derived from biomasses [54, 55]. A variety of bio-based solvents are nowadays produced via fermentation technology and have shown success as media for biocatalytic reactions [56]. It must be noted, however, that their bio-based origin does not assure their greenness, since considerations on their obtainment and disposal should be taken into account. Some commercially available bio-solvents are lactic acid esters, fatty acid esters, glycerol derivatives (triacetin, solketal, glycerol formal), 2-methyltetrahydrofuran (2-MeTHF, obtained from agricultural by-products) [43, 57, 58].
Due to current research on “green solvents” with reduced impact on the environment, more sustainable non-aqueous processes can be developed. Solvent recycling and the adoption of green solvent can significantly contribute to reducing costs, enhancing safety during handling, and prioritizing environmental friendliness in industrial operations.
Automation
The concept of “automation” in organic chemistry, especially related to continuous manufacturing, was extensively developed by Prof. Ley and his research group for the obtainment of efficient, sustainable and innovative processes. The main aspects concern work-up procedures, including purification steps, and in-line analysis which are typically considered a bottleneck in continuous processing.
Solid supported reagents consisting in reactive species associated with heterogeneous supports have dominated product purification under flow conditions [59]. Fulfilling chemical interactions (e.g., covalent bonds as well as weaker interactions such as ionic, electrostatic, hydrophobic bonds etc.) between impurities and the solid matrix, they should trap the undesired species obtaining the desired compound in the final flow stream. Due to their wide employment, a great number of differently functionalized scavengers are available on the market. However, they present some limitations: since solid-supported reagents cannot be recycled and reused continuously, their use needs the flow sequence interruption. As an example, “catch-and-release” procedures, where the undesired species are blocked via weak interactions (e.g., ionic bonds), require the addition of an exogenous reagent (e.g., acid or base solutions) for the recovery of the trapped molecule. Furthermore, the addition of various purification steps via scavenger technology during the process scale-up typically leads to scale-dependent phenomena such as dispersion and diffusion, so a careful design of the flow process is needed before their employment. Although numerous efforts have been made for the development of various tools to enhance the work-up procedure automation, especially taking into account the reduction of environmental burden, flow synthesis is typically followed by down-stream discontinuous batch purifications. This is particularly true when complex mixture of products possessing the same functional groups are generated [60,61,62]. Just few examples of in-line purification procedures involving multi-step chromatography or simulated moving bed (SMB) chromatography have been reported [63,64,65]. In this context, the work presented by Thomson and collaborators about the in-line addition of normal- /reversed-phase flash chromatography suggested guidelines for the selection of reactions whose products can be continuously isolated with high purity [62]. Even though these techniques present a great potential for their connection with flow processes, they also show several drawbacks in terms of high complexity, costs, and versatility.
Although liquid–liquid extractions are fundamental as well as common purification strategies also at industrial scale [66], they are considered among the most manually intensive and time/space consuming procedures. Furthermore, the massive use of organic solvents makes them also environmentally-unfriendly. Therefore, significant efforts have been spent into the development of devices to automatically perform such a procedure minimizing the amount of solvent. Among the most employed and commercially available plug-and-play modular units the Zaiput liquid–liquid separator is noteworthy (www.zaiput.com). This device relies on a quick mixing of the immiscible solvents using PTFE tubes and a subsequent separation due to solvent interactions with a membrane (one phase presents affinity for the membrane filling its pores while the other one is repelled). Different type of membranes (e.g., hydrophilic/hydrophobic materials, various pore size etc.) are now available on the market for the effective separation of a number of solvents.
Examples of in-line evaporation and distillation have also been reported [67]. Distillation seems to be particular interesting since it can allow for in-line purification, solvent exchange, and solvent recovery and recycling.
Although the promising continuous advancements of flow chemistry technology, there are still many hurdles to overcome especially in the handling of solids, which typically cause pressure increasing making precipitation difficult to manage under continuous conditions. Among the most common procedures to avoid clogging events, flushing the reactor with adequate solvents as well as sonication of the reagents while they are flowing through the reactors are the most employed [68, 69]. Alternative strategies have been also reported but they are tailor-made for specific systems, and a common solution is not available yet [70, 71].
In-line quality control devices and robust process analytical technology (PAT) are mandatory to comply with the regulatory obligations, especially for the continuous manufacturing of APIs (Active Pharmaceutical Ingredients). The work of Prof. Kappe and colleagues about the use of microreactor connected to an in-line NMR and FTIR instrument for the real-time monitoring of the reaction mixture is noteworthy. The obtained data are then processed by a chemometric model for the optimization of 7 reaction parameters without any human intervention. However, this automated process is not easily usable for the preparation of APIs since the necessary amount of precursor may be the limiting factor [72]. Another very good example is reported by Cronin and coworkers with the development of a robotic platform connected to an intuitive software able to drive the different synthetic steps: reaction, work-up, and product purification. As a proof of concept, the fully automated synthesis of the antihistamine diphenydramine hydrochloride, the anticonvulsant rufinamide and sildenafil used to treat erectile dysfunction were performed with comparable yields with respect to the classical synthetic procedures [73, 74]. In this context, the great work of the team led by Bourne in the development of autonomous continuous platforms combining both in-line Bayesian and HPLC analyses, dramatically speed-up the optimization of pharmaceutical processes [75].
Although these systems are usable at academic level, they are not suited for industrial synthesis yet, since any equipment employed at industrial level must be supplied with specific documents to allow its qualification and validation prior to use. Furthermore, contingency plans regarding device failure or maintenance should be carefully evaluated and designed.
Biocatalytic process intensification
As mentioned above, one of the advantages of immobilized biocatalysts is their employment under continuous conditions. The compartmentalization of the immobilized enzymes into packed-bed reactors (PBRs) allows for a high amount of catalyst to be accumulated in a small space where the substrate flows in a controlled manner. Moreover, mass and heat transfer have been demonstrated to be more efficient with respect to conventional batch mode, allowing for faster and highly-productive reactions [76]. One of our most representative examples is the multi-scale production of melatonin and its analogues through direct acetylation of the commercially available 5-metoxy-tryptamine mediated by MsAcT [34]. A small reactor containing less than 2 mg (1.8 g of packed imm-MsAcT; 1.2 mL reactor volume) of the enzyme could handle substrate at 0.5 M (95 g/L) with a production of the desired compound of 36 g/day. The system was integrated with an in-line extraction to allow for the collection and reuse of both the organic solvent and aqueous phase (Fig. 3). The same procedure has been utilized for the process intensification for the production of natural esters as flavor compounds with excellent yields and residence times, highlighting the versatility and sustainability of the developed methodology (Fig. 3) [77]. Different PBRs can be also sequentially connected to perform multi-step biotransformations increasing the complexity of the cascade [32]. The beauty of this system is its tunability and flexibility: by changing the cartridges or the way to combine them, different products can be generated employing the same starting materials. In some cases, artificial metabolisms simulating the extremely efficient cell pathways have been developed demonstrating the use of flow biocatalysis as an artificial cell-factory [9].
Novel combined approaches
The progress in our understanding of enzymes and their potential as catalysts together with the global goal to enhance sustainability, reducing waste and emissions has defined a novel status quo where biocatalysis is becoming a strong ally for both chemists and engineers with a variety of alternatives now available. In this context, flow biocatalysis is becoming an attractive technique for both academics and industrials due to the small equipment footprint, the highest reaction selectivity, mild operational conditions, as well as the possibility to combine it with both chemical procedures (i.e., chemo-enzymatic reactions) and novel green techniques (e.g., photochemistry), thus bringing chemical processes to the next level in terms of system versatility and sustainability. In particular, photo-biocatalysis represents a novel chapter and just few examples have been reported, mainly regarding light-driven enzymes, light-activated cofactor recycling as well as the use of light-dependent organisms. A series of enzymes showing a different or broader reaction spectrum under light irradiation have been described [78, 79]. Moreover, various publications reported the light-driven activation of redox-enzymes especially employing photosensitizers or mediators for electron transfer. In this context, the photochemical regeneration of cofactors plays an important role, since it is an effective and simple approach to integrate photo- and biocatalysis. However, up to now, neither photochemical activation nor light-driven cofactor regeneration have become standard procedures. This is mainly due to the poor transfer kinetics of photo-excited electrons to enzyme, which produced low TTN (i.e., total turnover number) and TOF (i.e., turnover frequency) of the photo- or biocatalyst. Another problem consists in the generation of strong oxidants and very reactive free radicals which may conduct to side-reactions and lead to enzyme inactivation [80]. However, these limitations can be overcome by an increasing number of different compounds employable as photosensitizers which can allow for a more efficient electron transfer as well as by enzyme modification via protein engineering to simplify the electron acceptance, thus improving TTN and TOF [81]. In this context, the possibility to incorporate non-natural amino acids in the enzyme catalytic site enabling the employment of non-native cofactors seems to be noteworthy [82,83,84].
As an alternative to these artificial systems a new branch of photo-biocatalysis has emerged using whole cell such as photo-autotrophic organisms (i.e., cyanobacteria or algae) where light is employed as energy source for enzymes: the photosystem converts energy deriving from light into redox equivalents, while the cell presents highly specialized electron transport chains, mechanisms for the control of reactive species, and the regeneration of damaged parts of the system [85].
A challenge for applying photo-biocatalytic reactions is their employment on a large scale. In fact most of the discussed reactions are proposed on analytical scale. A logical step will be the demonstration of the feasibility of this technique when applied to larger volumes and concentrations. However, in contrast with classical biotransformations which typically allow for straightforward upscaling, parameters such as the light intensity or light penetration depth cannot be scaled easily [86, 87]. This may be addressed with novel led photoreactors which are now becoming available for continuous processing [86], although also these systems do not allow for a fast and reliable evaluation of different illumination and reaction conditions, as only a single sample can be tested at a time [87]. Taking all these considerations into account, to make photo-biocatalysis a robust technique widely employable it is crucial to develop efficient photoreactors for large scale production as well as standardize as much as possible small scale reactions. Basic concepts have been up to now developed but need to be extended in a way that novel reactions and concepts could come up.
Conclusions
Within the scope of Green Chemistry, a minimization of waste deriving from chemical reactions needs to be taken into account, as well as cost-efficient processes compatible with the time scale industry. Considering the benefits of flow biocatalysis, this technique can be useful for a greener and cost-effective production of chemicals in our daily life, especially regarding its compliance with US and European regulation (i.e., FDA and EMA) in terms of preparation of natural molecules. In fact, processing natural compounds with biocatalytic approaches will allow for the commercialization of the final product as natural too, thus increasing its market value. Despite the combination of biocatalytic approaches with in continuous strategies is nowadays considered at the forefront of the sustainability, the calculation of the environmental footprint of these processes (e.g. Green Chemistry Metrics, LCA assessment) should be systematically applied since data is often missing in the available literature. Although many APIs have been already bio-synthesized at lab scale, and their preparation through flow chemistry technique would dramatically impact on production costs, the development of flow biocatalysis at industrial level needs to be further implemented to become a widely employed technology. A key point working towards the development of biocompatible systems is the combination of biocatalysis with traditional chemistry, and novel green techniques such as photocatalysis, thus generating innovative and versatile multi-step syntheses. Since light can be considered as a unique environmentally compatible option to initiate reactions, its coupling with biocatalysis, especially under flow conditions, will open the path to novel reactions and concepts strengthening and accelerating the uptake of flow biocatalysis to industry. Considering the high sustainability, selectivity and flexibility of flow biocatalysis together with the possibility of integrating this with old fashion and innovative techniques a brighter greener and cost-efficient future for chemical production will be possible.
References
Heckmann CM, Paradisi F (2020) Looking back: A short history of the discovery of enzymes and how they became powerful chemical tools. ChemCatChem 12:6082–6102. https://doi.org/10.1002/cctc.202001107
Wu S, Snajdrova R, Moore JC et al (2021) Biocatalysis: Enzymatic synthesis for industrial applications. Angewandte Chemie - International Edition 60:88–119. https://doi.org/10.1002/anie.202006648
Huffman MA, Fryszkowska A, Alvizo O et al (1979) (2020) Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 368:1255–1259. https://doi.org/10.1126/SCIENCE.ABC1954
Savile CK, Janey JM, Mundorff EC et al (1979) (2010) Biocatalytic asymmetric synthesis of sitagliptin manufacture. Science 329:305–310. https://doi.org/10.1126/science.1188934
Melton L (2023) So sweet, no spike. Nat Biotechnol 41:883–885. https://doi.org/10.1038/s41587-023-01860-2
Benítez-Mateos AI, Contente ML, Roura Padrosa D, Paradisi F (2021) Flow biocatalysis 101: Design, development and applications. React Chem Eng 6:599–611. https://doi.org/10.1039/d0re00483a
Leemans Martin L, Peschke T, Venturoni F, Mostarda S (2020) Pharmaceutical industry perspectives on flow chemocatalysis and biocatalysis. Curr Opin Green Sustain Chem 25:100350. https://doi.org/10.1016/j.cogsc.2020.04.011
Basso A, Serban S (2019) Industrial applications of immobilized enzymes—A review. Molecular Catalysis 479:110607. https://doi.org/10.1016/j.mcat.2019.110607
Donzella S, Colacicco A, Nespoli L, Contente ML (2022) Mimicking natural metabolisms: Cell-free flow preparation of dopamine. ChemBioChem 23.https://doi.org/10.1002/cbic.202200462
Crotti M, Robescu MS, Bolivar JM et al (2023) What’s new in fl ow biocatalysis ? A snapshot of 2020–2022:1–10. https://doi.org/10.3389/fctls.2023.1154452
Sheldon RA (2012) Fundamentals of green chemistry: Efficiency in reaction design. Chem Soc Rev 41:1437–1451. https://doi.org/10.1039/c1cs15219j
Sheldon RA, Bode ML, Akakios SG (2022) Metrics of green chemistry: Waste minimization. Curr Opin Green Sustain Chem 33:100569. https://doi.org/10.1016/J.COGSC.2021.100569
Alcántara AR, Domínguez de María P, Littlechild JA et al (2022) Biocatalysis as key to sustainable industrial chemistry. Chemsuschem 15:e202102709
Becker M, Ziemińska-Stolarska A, Markowska D, et al (2023) Comparative life cycle assessment of chemical and biocatalytic 2’3’-Cyclic GMP-AMP Synthesis. ChemSusChem 16.https://doi.org/10.1002/cssc.202201629
Betancur-Ramírez KJ, Meneses-Jácome A, Ruiz-Colorado AA, Gallego-Suárez D (2021) Life cycle assessment of an alternative enzymatic-biological treatment for effluents from industrial processing of potatoes. J Clean Prod 324. https://doi.org/10.1016/j.jclepro.2021.129151
Tufvesson P, Fu W, Jensen JS, Woodley JM (2010) Process considerations for the scale-up and implementation of biocatalysis. Food Bioprod Process 88:3–11. https://doi.org/10.1016/j.fbp.2010.01.003
Lima-Ramos J, Tufvesson P, Woodley JM (2014) Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process Synth 3:195–213. https://doi.org/10.1515/gps-2013-0094
Calvo-Flores FG (2009) Sustainable chemistry metrics. Chemsuschem 2:905–919. https://doi.org/10.1002/cssc.200900128
Curzons AD, Constable DJC, Mortimer DN, Cunningham VL (2001) So you think your process is green, how do you know? - Using principles of sustainability to determine what is green - A corporate perspective. In: Green Chemistry. Royal Society of Chemistry, pp 1–6
Sheldon RA (2018) Metrics of green chemistry and sustainability: Past, Present, and Future. ACS Sustain Chem Eng 6:32–48
Gerbens-Leenes W, Hoekstra AY, Van Der Meer TH (2009) The water footprint of bioenergy. PNAS 10219–10223
Ma SK, Gruber J, Davis C et al (2010) A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem 12:81–86. https://doi.org/10.1039/b919115c
Hudlicky T, Frey DA, Koroniak L, Claeboe CD, Brammer LE (1999) Toward a ‘reagent-free’ synthesis. Food Chem 57–59
Jimenez-Gonzalez C, Ponder CS, Broxterman QB, Manley JB (2011) Using the right green yardstick: Why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes. Org Process Res Dev 15:912–917. https://doi.org/10.1021/op200097d
Sheldon RA (1996) Selective catalytic synthesis of fine chemicals: opportunities and trends. J Mol Catal A Chem 107:75–83
Constable DJC (2021) Perspective Green and sustainable chemistry-The case for a systems-based, interdisciplinary approach. iScience 24. https://doi.org/10.1016/j.isci
DeSimone JM (1979) (2002) Practical approaches to green solvents. Science 297:799–803
Donzella S, Fumagalli A, Arioli S, et al (2022) Recycling food waste and saving water: Optimization of the fermentation processes from cheese whey permeate to yeast oil. Fermentation 8. https://doi.org/10.3390/fermentation8070341
Bolivar JM, López-Gallego F (2020) Characterization and evaluation of immobilized enzymes for applications in flow reactors. Curr Opin Green Sustain Chem 25:100349. https://doi.org/10.1016/j.cogsc.2020.04.010
Romero-Fernández M, Paradisi F (2020) Protein immobilization technology for flow biocatalysis. Curr Opin Chem Biol 55:1–8. https://doi.org/10.1016/j.cbpa.2019.11.008
Pinto A, Contente ML, Tamborini L (2020) Advances on whole-cell biocatalysis in flow. Curr Opin Green Sustain Chem 25:100343. https://doi.org/10.1016/j.cogsc.2020.04.004
Contente ML, Paradisi F (2018) Self-sustaining closed-loop multienzyme- mediated conversion of amines into alcohols in continuous reactions. Nat Catal 1:452–459. https://doi.org/10.1038/s41929-018-0082-9
Romero-Fernandez M, Paradisi F (2020) General overview on immobilization techniques of enzymes for biocatalysis. In: Sons JW& (ed) Catalyst immobilization Eds. M Benagilia, A. Puglisi, pp 409–435
Contente ML, Farris S, Tamborini L et al (2019) Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: A five-minute intensified process. Green Chem 21:3263–3266. https://doi.org/10.1039/c9gc01374a
Mateo C, Fernandez-Lorente G, Cortes E et al (2001) One-step purification, Covalent immobilization, and additional stabilization of poly-his-tagged proteins using novel heterofunctional chelate-epoxy supports. Biotechnol Bioeng 73:253–258. https://doi.org/10.1002/bit.1058
Planchestainer M, Contente ML, Cassidy J et al (2017) Continuous flow biocatalysis : production and in-line puri fi cation of amines by immobilised transaminase from Halomonas elongata †. Green Chem 19:372–375. https://doi.org/10.1039/c6gc01780k
Velasco-Lozano S, Santiago-Arcos J, Mayoral JA, López-Gallego F (2020) Co-immobilization and colocalization of multi-enzyme systems for the cell-free biosynthesis of aminoalcohols. ChemCatChem 12:3030–3041. https://doi.org/10.1002/cctc.201902404
Wheeldon I, Minteer SD, Banta S et al (2016) Substrate channelling as an approach to cascade reactions. Nat Chem 8:299–309. https://doi.org/10.1038/nchem.2459
Schoffelen S, Van Hest JCM (2013) Chemical approaches for the construction of multi-enzyme reaction systems. Curr Opin Struct Biol 23:613–621. https://doi.org/10.1016/j.sbi.2013.06.010
Colacicco A, Catinella G, Pinna C, et al (2023) Flow bioprocessing of citrus glycosides for high-value aglycone preparation. Catal Sci Technol 4348–4352. https://doi.org/10.1039/d3cy00603d
Roura Padrosa D, Marchini V, Paradisi F (2021) CapiPy: Python-based GUI-application to assist in protein immobilization. Bioinformatics 37:2761–2762. https://doi.org/10.1093/bioinformatics/btab030
Eissen M, Weiß M, Brinkmann T, Steinigeweg S (2010) Comparison of two alternative routes to an enantiomerically pure β-Amino acid. Chem Eng Technol 33:629–637. https://doi.org/10.1002/ceat.201000046
Welton T (2015) Solvents and sustainable chemistry. Proc Royal Soc A Math Phys Eng Sci 471(2183):20150502
Gani R, Gómez PA, Folić M et al (2008) Solvents in organic synthesis: Replacement and multi-step reaction systems. Comput Chem Eng 32:2420–2444. https://doi.org/10.1016/j.compchemeng.2008.01.006
van Schie MMCH, Spöring JD, Bocola M et al (2021) Applied biocatalysis beyond just buffers - From aqueous to unconventional media. Opt Guidel Green Chem 23:3191–3206
de María PD, Hollmann F (2015) On the (Un)greenness of biocatalysis: Some challenging figures and some promising options. Front Microbiol 6
Chanquia SN, Benfeldt FV, Petrovai N, et al (2022) Immobilization and application of fatty acid photodecarboxylase in deep eutectic solvents. ChemBioChem 23.https://doi.org/10.1002/cbic.202200482
Uysal D, Karadaş C, Kara D (2017) Ionic liquid dispersive liquidliquid microextraction method for the determination of irinotecan, an anticancer drug, in water and urine samples using UV-Vis Spectrophotometry. J AOAC Int. https://doi.org/10.5740/jaoacinct.16-0219
Hernáiz MJ, Alcántara AR, García JI, Sinisterra JV (2010) Applied biotransformations in green solvents. Chem Eur J 16:9422–9437
Marques MPC, Lourenço NMT, Fernandes P, Carvalho de CCCR, (2012) Green solvents for biocatalysis. Green Solvents I: Properties and Applications in Chemistry. Springer, Netherlands, pp 121–146
Hobbs HR, Thomas NR (2007) Biocatalysis in supercritical fluids, in fluorous solvents, and under solvent-free conditions. Chem Rev 107:2757–2785
Cantone S, Hanefeld U, Basso A (2007) Biocatalysis in non-conventional media—ionic liquids, supercritical fluids and the gas phase. Green Chem 9:954–997. https://doi.org/10.1039/b618893a
da Silva RPFF, Rocha-Santos TAP, Duarte AC (2016) Supercritical fluid extraction of bioactive compounds. TrAC - Trends Analytic Chem 76:40–51
Clark JH, Farmer TJ, Hunt AJ, Sherwood J (2015) Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources. Int J Mol Sci 16:17101–17159
Domínguez de María P (2023) Green solvents and biocatalysis: A bigger picture. EFB Bioecon J 3:100056. https://doi.org/10.1016/j.bioeco.2023.100056
Byrne F, Jin S, Sherwood J, et al (2017) 3 Solvents from waste
Esteban J, Vorholt AJ, Leitner W (2020) An overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: A rational selection of solvents using COSMO-RS and selection guides. Green Chem 22:2097–2128. https://doi.org/10.1039/c9gc04208c
Clark JH, Macquarrie DJ, Sherwood J (2012) A quantitative comparison between conventional and bio-derived solvents from citrus waste in esterification and amidation kinetic studies. Green Chem 14:90–93. https://doi.org/10.1039/c1gc16299c
Ley SV, Baxendale IR, Bream RN et al (2000) Multi-step organic synthesis using solid-supported reagents and scavengers: A new paradigm in chemical library generation. J Chem Soc Perkin 1:3815–4195. https://doi.org/10.1039/b006588i
Agostino FJ, Krylov SN (2015) Advances in steady-state continuous-flow purification by small-scale free-flow electrophoresis. TrAC - Trends Anal Chem 72:68–79. https://doi.org/10.1016/j.trac.2015.03.023
Bana P, Örkényi R, Lövei K et al (2017) The route from problem to solution in multistep continuous flow synthesis of pharmaceutical compounds. Bioorg Med Chem 25:6180–6189. https://doi.org/10.1016/j.bmc.2016.12.046
Newton S, Carter CF, Pearson CM et al (2014) Accelerating spirocyclic polyketide synthesis using flow chemistry. Angewandte Chemie - International Edition 53:4915–4920. https://doi.org/10.1002/anie.201402056
Horosanskaia E, Triemer S, Seidel-Morgenstern A, Lorenz H (2019) Purification of artemisinin from the product solution of a semisynthetic reaction within a single crystallization step. Org Process Res Dev 23:2074–2079. https://doi.org/10.1021/acs.oprd.9b00175
Gilmore K, Kopetzki D, Lee JW et al (2014) Continuous synthesis of artemisinin-derived medicines. Chem Commun 50:12652–12655. https://doi.org/10.1039/c4cc05098c
Sreedhar B, Shen B, Li H et al (2017) Optimal design of integrated SMB-crystallization hybrid separation process using a binary solvent. Org Process Res Dev 21:31–43. https://doi.org/10.1021/acs.oprd.6b00294
Towler G, Sinnott RAY (2013) Principles, Practice and economics of plant and process design. In: Chemical Engineering Design
Deadman BJ, Battilocchio C, Sliwinski E, Ley SV (2013) A prototype device for evaporation in batch and flow chemical processes. Green Chem 15:2050–2055. https://doi.org/10.1039/c3gc40967h
Kelly CB, Lee C, Leadbeater NE (2011) An approach for continuous-flow processing of reactions that involve the in situ formation of organic products. Tetrahedron Lett 52:263–265. https://doi.org/10.1016/j.tetlet.2010.11.027
Sedelmeier J, Ley SL, Baxendale IR, Baumann M (2010) KMnO4-Mediated oxidation as a continuous flow process. Org Lett 12:3618–3621. https://doi.org/10.1016/j.tet.2010.04.092
Amador C, Gavriilidis A, Angeli P (2004) Flow distribution in different microreactor scale-out geometries and the effect of manufacturing tolerances and channel blockage. Chem Eng J 101:379–390. https://doi.org/10.1016/j.cej.2003.11.031
Mascia S, Heider PL, Zhang H et al (2013) End-to-end continuous manufacturing of pharmaceuticals: Integrated synthesis, purification, and final dosage formation. Angewandte Chemie - International Edition 52:12359–12363. https://doi.org/10.1002/anie.201305429
Sagmeister P, Ort FF, Jusner CE et al (2022) Autonomous multi-step and multi-objective optimization facilitated by real-time process analytics. Adv Sci 9:1–9. https://doi.org/10.1002/advs.202105547
Hessam S, Craven M, Leonov AI et al (1979) (2020) A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370:101–108. https://doi.org/10.1126/science.abc2986
Steiner S, Wolf J, Glatzel S, et al (2019) Organic synthesis in a modular robotic system driven by a chemical programming language. Science (1979) 363. https://doi.org/10.1126/science.aav2211
Clayton AD, Pyzer-Knapp EO, Purdie M, et al (2023) Bayesian self-optimization for telescoped continuous flow synthesis. AngewandteChemie - International Edition 62. https://doi.org/10.1002/anie.202214511
Britton J, Raston CL (2017) Multi-step continuous-flow synthesis. Chem Soc Rev 46:1250–1271. https://doi.org/10.1039/c6cs00830e
Contente ML, Tamborini L, Molinari F, Paradisi F (2020) Aromas flow: eco-friendly, continuous, and scalable preparation of flavour esters. J Flow Chem 10:235–240. https://doi.org/10.1007/s41981-019-00063-8
Lee SH, Choi DS, Kuk SK, Park CB (2018) Photobiocatalysis: Activating redox enzymes by direct or indirect transfer of photoinduced electrons. Angewandte Chemie - International Edition 57:7958–7985. https://doi.org/10.1002/anie.201710070
Emmanuel MA, Greenberg NR, Oblinsky DG, Hyster TK (2016) Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540:414–417. https://doi.org/10.1038/nature20569
Hollmann F, Arends IWCE, Buehler K (2010) Biocatalytic redox reactions for organic synthesis: Nonconventional regeneration methods. ChemCatChem 2:762–782. https://doi.org/10.1002/cctc.201000069
Schmermund L, Jurkaš V, Özgen FF et al (2019) Photo-Biocatalysis: Biotransformations in the presence of light. ACS Catal 9:4115–4144. https://doi.org/10.1021/acscatal.9b00656
Drienovská I, Mayer C, Dulson C, Roelfes G (2018) A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue. Nat Chem 10:946–952. https://doi.org/10.1038/s41557-018-0082-z
Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79:413–444. https://doi.org/10.1146/annurev.biochem.052308.105824
Li Y, Dalby PA (2022) Engineering of enzymes using non-natural amino acids. Biosci Rep 42:1–15. https://doi.org/10.1042/BSR20220168
Köninger K, Gómez Baraibar Á, Mügge C et al (2016) Recombinant cyanobacteria for the asymmetric reduction of C=C bonds fueled by the biocatalytic oxidation of water. Angewandte Chemie - International Edition 55:5582–5585. https://doi.org/10.1002/anie.201601200
Heining M, Buchholz R (2015) Photobioreactors with internal illumination - A survey and comparison. Biotechnol J 10:1131–1137. https://doi.org/10.1002/biot.201400572
Glemser M, Heining M, Schmidt J et al (2016) Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives. Appl Microbiol Biotechnol 100:1077–1088. https://doi.org/10.1007/s00253-015-7144-6
Acknowledgements
M.L.C. acknowledges funding from University of Milan (Piano di Sostegno UNIMI Linea 2-2021) (Italy) through the project W-BioFlow “From agro-food waste to valuable bioactives through flow biocatalysis”.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article Highlights
- The combination between biocatalysis and flow facilities represents an innovative tool to increase the sustainability of the chemical processes.
- The use of immobilized biocatalysts under continuous conditions enhances the catalyst efficiency and stability.
- Merging biocatalysis and novel green techniques, versatile multi-step syntheses can be performed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donzella, S., Contente, M.L. The joint effort of enzyme technology and flow chemistry to bring biocatalytic processes to the next level of sustainability, efficiency and productivity. J Flow Chem 14, 85–96 (2024). https://doi.org/10.1007/s41981-023-00286-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-023-00286-w