Abstract
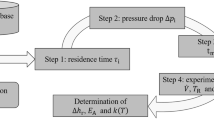
In this paper, a microsystem integrated with infrared thermography is developed to measure the kinetics of fast exothermic reactions. The hydrolysis of ethyl acetate is chosen as the model reaction to verify the technique. The activation energy of this reaction is successfully obtained, which agrees well with the literature value. Except for the general method of collecting data from several typical points of the infrared radiation (IR) picture, a strategy called image analysis is applied to obtain the temperature profiles along the reactor by collecting data from the whole IR picture. With the image analysis, this system can generate time-series kinetic data in a single experiment, which is usually challenging for a continuous flow system. The reaction enthalpy of classic neutralization reaction and kinetic parameter of hydrolysis are determined again using the image analysis, showing fewer deviations from literature values. The image analysis technique could provide much more reliable and accurate results with higher efficiency.








Similar content being viewed by others
References
Zhang J, Wang K, Lin X, Lu Y, Luo G (2014) Intensification of fast exothermic reaction by gas agitation in a microchemical system. AICHE J 60(7):2724–2730
Gemoets HP, Su Y, Shang M, Hessel V, Luque R, Noel T (2016) Liquid phase oxidation chemistry in continuous-flow microreactors. Chem Soc Rev 45(1):83–117. https://doi.org/10.1039/c5cs00447k
Zhou F, Zhang B, Yao C, Zhu K, Yang M, Chen G (2016) Cyclization of pseudoionone catalyzed by sulfuric acid in a microreactor. Chem Eng Technol 39(5):849–856. https://doi.org/10.1002/ceat.201500670
Knapkiewicz P, Skowerski K, Jaskólska DE, Barbasiewicz M, Olszewski TK (2012) Nitration under continuous flow conditions: convenient synthesis of 2-isopropoxy-5-nitrobenzaldehyde, an important building block in the preparation of nitro-substituted Hoveyda–Grubbs metathesis catalyst. Org Process Res Dev 16(8):1430–1435. https://doi.org/10.1021/op300116j
Zhang C, Zhang J, Luo G (2016) Kinetic study and intensification of acetyl guaiacol nitration with nitric acid—acetic acid system in a microreactor. J Flow Chem 6(4):309-314. https://doi.org/10.1556/1846.2016.00011
Wang K, Lu YC, Xia Y, Shao HW, Luo GS (2011) Kinetics research on fast exothermic reaction between cyclohexanecarboxylic acid and oleum in microreactor. Chem Eng J 169(1–3):290–298. https://doi.org/10.1016/j.cej.2011.02.072
Schneider MA, Stoessel F (2005) Determination of the kinetic parameters of fast exothermal reactions using a novel microreactor-based calorimeter. Chem Eng J 115(1–2):73–83. https://doi.org/10.1016/j.cej.2005.09.019
Zogg A, Stoessel F, Fischer U, Hungerbühler K (2004) Isothermal reaction calorimetry as a tool for kinetic analysis. Thermochim Acta 419(1–2):1–17. https://doi.org/10.1016/j.tca.2004.01.015
Glotz G, Knoechel DJ, Podmore P, Gruber-Woelfler H, Kappe CO (2017) Reaction calorimetry in microreactor environments—measuring heat of reaction by isothermal heat flux calorimetry. Org Process Res Dev 21(5):763–770. https://doi.org/10.1021/acs.oprd.7b00092
Andreozzi R, Caprio V, Di Somma I, Sanchirico R (2006) Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J Hazard Mater 134(1–3):1–7. https://doi.org/10.1016/j.jhazmat.2005.10.037
McMullen JP, Jensen KF (2011) Rapid determination of reaction kinetics with an automated microfluidic system. Org Process Res Dev 15(2):398–407. https://doi.org/10.1021/op100300p
Reichmann F, Millhoff S, Jirmann Y, Kockmann N (2017) Reaction calorimetry for exothermic reactions in plate-type microreactors using seebeck elements. Chem Eng Technol 40(11):2144–2154. https://doi.org/10.1002/ceat.201700419
Gomez MV, Rodriguez AM, de la Hoz A, Jimenez-Marquez F, Fratila RM, Barneveld PA, Velders AH (2015) Determination of kinetic parameters within a single nonisothermal on-flow experiment by nanoliter NMR spectroscopy. Anal Chem 87(20):10547–10555. https://doi.org/10.1021/acs.analchem.5b02811
Sans V, Cronin L (2016) Towards dial-a-molecule by integrating continuous flow, analytics and self-optimisation. Chem Soc Rev 45(8):2032–2043. https://doi.org/10.1039/c5cs00793c
Halder R, Lawal A, Damavarapu R (2007) Nitration of toluene in a microreactor. Catal Today 125(1–2):74–80. https://doi.org/10.1016/j.cattod.2007.04.002
Zhang J, Burklé-Vitzthum V, Marquaire PM (2012) NO2-promoted oxidation of methane to formaldehyde at very short residence time – part II: kinetic modeling. Chem Eng J 197:123–134. https://doi.org/10.1016/j.cej.2012.05.013
Keybl J, Jensen KF (2011) Microreactor system for high-pressure continuous flow homogeneous catalysis measurements. Ind Eng Chem Res 50(19):11013–11022. https://doi.org/10.1021/ie200936b
Buffone C, Sefiane K (2004) IR measurements of interfacial temperature during phase change in a confined environment. Exp Thermal Fluid Sci 29(1):65–74. https://doi.org/10.1016/j.expthermflusci.2004.02.004
Hetsroni G, Mosyak A, Pogrebnyak E, Rozenblit R (2011) Infrared temperature measurements in micro-channels and micro-fluid systems. Int J Therm Sci 50(6):853–868. https://doi.org/10.1016/j.ijthermalsci.2011.01.006
Ravey C, Pradere C, Regnier N, Batsale J-C (2012) New temperature field processing from IR camera for velocity, thermal diffusivity and calorimetric non-intrusive measurements in microfluidics systems. Quant InfraRed Thermogr J 9(1):79–98. https://doi.org/10.1080/17686733.2012.682878
Haber J, Kashid MN, Borhani N, Thome J, Krtschil U, Renken A, Kiwi-Minsker L (2013) Infrared imaging of temperature profiles in microreactors for fast and exothermic reactions. Chem Eng J 214:97–105. https://doi.org/10.1016/j.cej.2012.10.021
Ravey C, Pradere C, Regnier N, Batsale JC (2015) Study of phase change and Supercooling in micro-channels by infrared thermography. Exp Heat Transfer 29(2):266–283. https://doi.org/10.1080/08916152.2014.973980
Zhang J, Zhang C, Liu G, Luo G (2016) Measuring enthalpy of fast exothermal reaction with infrared thermography in a microreactor. Chem Eng J 295:384–390
Ashauer M, Ende J, Glosch H, Haffner H, Hiltmann K (1997) Thermal characterization of microsystems by means of high-resolution thermography. Microelectron J 28(3):327–335. https://doi.org/10.1016/s0026-2692(96)00036-5
Zhang J, Wang K, Lu Y, Luo G (2010) Characterization and modeling of micromixing performance in micropore dispersion reactors. Chem Eng Process Process Intensif 49(7):740–747
Johnson RE, Biltonen RL (1975) Determination of reaction rate parameters by flow microcalorimetry. J Am Chem Soc 97(9):2349–2355. https://doi.org/10.1021/ja00842a007
Roux A, Perron G, Picker P, Desnoyers JE (1980) Enthalpies of reaction and reaction rates by flow microcalorimetry: Ester hydrolysis in basic medium. J Solut Chem 9(1):59–73. https://doi.org/10.1007/bf00650137
Bamford CH, Tipper CFH (1972) Comprehensive chemical kinetics. Elsevier, Amsterdam
Reichmann F, Vennemann K, Frede TA, Kockmann N (2019) Mixing time scale determination in microchannels using reaction calorimetry. Chemie Ingenieur Technik. https://doi.org/10.1002/cite.201800169
Acknowledgements
We gratefully acknowledge the supports of the National Natural Science Foundation of China (21991103, U1463208), Tsinghua University Initiative Scientific Research Program (2019Z08QCX02) and the State Key Laboratory of Chemical Engineering (SKL-ChE-17T01) on this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 882 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, J. & Luo, G. Kinetics determination of fast exothermic reactions with infrared thermography in a microreactor. J Flow Chem 10, 219–226 (2020). https://doi.org/10.1007/s41981-019-00071-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00071-8