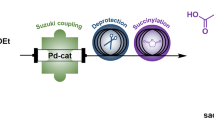
Abstract
Valsartan is a potent, orally active angiotensin II receptor blocker and is widely used in the treatment of hypertension and chronic heart failure. Herein, we present an approach for the continuous synthesis of a late-stage precursor of valsartan in three steps. The applied synthetic route involves N-acylation, Suzuki-Miyaura cross-coupling and methyl ester hydrolysis. After optimization of the individual steps in batch, they were successfully transferred to continuous flow processes employing different reactor designs. The first step of the synthetic route (N-acylation) as well as the third step (methyl ester hydrolysis) are performed in coil reactor setups. The key step of the reaction cascade (Suzuki-Miyaura cross-coupling) is catalyzed by a heterogeneous palladium-substituted cerium-tin-oxide with the molecular formula Ce0.20Sn0.79Pd0.01O2-δ. The catalyst particles are implemented in an in-house developed packed-bed reactor, which features an HPLC column as fixed-bed. The combination of the individual reaction modules facilitates the consecutive performance of the three reaction steps. Using the developed multistep continuous setup, the targeted valsartan precursor was obtained with up to 96% overall yield.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO), in 2015 about 31% of deaths were ascribable to cardiovascular diseases, thus being the leading cause of death globally. Especially people suffering from high blood pressure belong to the risk group to develop cardiovascular complaints. Therefore, a number of different medications for the treatment of hypertension are available on the market [1, 2]. One popular therapy for lowering the patients’ blood pressure is the administration of the non-peptide angiotensin II receptor blocker valsartan 1. The active pharmaceutical ingredient (API) belongs to the class of sartans, which feature a biphenyl moiety as common structural unit. The biaryl scaffold plays a crucial role in the oral bioavailability as well as the binding of the API to the biological target [3, 4]. Valsartan 1 was first introduced onto the market as Diovan® by Novartis in 1997 [5, 6]. In the course of ensuing years, several other combination preparations followed, such as Diovan HCT® (+hydrochlorothiazide, 1998), Exforge® (+amlodipine, 2007), Exforge HCT® (+amlodipine+hydrochlorothiazide, 2009), Valtura® (+aliskiren, 2009) and Entresto® (+sacubitril, 2015) [7, 8]. Regarding the synthesis of the antihypertensive 1, it was first patented by Ciba-Geigy in 1991. The route involves the coupling of l-valine methyl ester 2 with biphenyl aldehyde 3a via reductive amination (Scheme 1). Subsequent acylation of the resulting intermediate 4 with valeryl chloride, triazole formation with an organotin reagent as well as saponification yielded the target molecule valsartan 1 [9].
Patented Ciba-Geigy synthesis of valsartan [9]
However, the Ciba-Geigy route to 1 offered plenty of room for improvement. The use of a highly toxic tin reagent led to contamination of the final API and the process suffered from a poor overall yield of less than 10% [10]. Consequently, a substantial number of papers and patents focusing on novel synthesis strategies and optimization of the overall reaction process have been published [exemplarily 3, 11–20]. Alternative approaches for the formation of valsartan 1 include Ru-catalyzed C-H activation [17], Pd-catalyzed decarboxylative biaryl coupling [3], Zn-catalyzed Negishi reaction [18] and the use of trityl-protected tetrazole intermediates in the Pd-catalyzed Suzuki-Miyaura cross-coupling [14, 15]. Although differing considerably in terms of employed reagents, most strategies for the synthesis of valsartan 1 rely exclusively on traditional batch operations. Only very few papers deal with the synthesis of valsartan precursor compounds in continuous flow. Pandarus et al. reported the continuous Suzuki coupling of 2-chlorobenzonitrile 7a with 4-methyl-phenylboronic acid 8 using a heterogeneous sol-gel entrapped SiliaCat DPP-Pd catalyst. Its implementation in a packed bed microreactor facilitated formation of valsartan building block 3b (Scheme 2, a). Nevertheless, Pandarus et al. observed rapid catalyst deactivation due to the instability of the active Pd-species towards dissolved oxygen in the presence of the aryl chloride substrate 7a [21]. Likewise, Nagaki and his group performed the continuous synthesis of valsartan intermediate 3a. First, organoboron species 9 is generated using a two-step tubular flow microreactor system. Then, Suzuki cross-coupling of the obtained intermediate with 10 is performed in a Pd-substituted monolithic microreactor (Scheme 2, b). Parallelization of five of these microreactor setups using a flow distributor allowed them to synthesize valsartan intermediate 3a on a larger scale with a productivity of 184 mg h−1 [22]. However, to the best of our knowledge, there are no approaches for the continuous multistep synthesis of an advanced valsartan precursor reported in literature. Therefore, the development of a multistep continuous setup for the synthesis of a late-stage valsartan precursor was the objective of our work.
In contrast to traditionally employed batch operations, flow reactors have a lot of benefits including improved energy efficiency, reduced waste generation and increased safety. Therefore, the Federal Drug Administration (FDA) aims for application of continuous synthesis in pharmaceutical manufacturing [23, 24]. Also, recent recalls of valsartan 1 generics, contaminated with carcinogenic N-nitrosodimethylamine, highlight the need for alternative synthesis methods to obtain safe and pure APIs [25]. Therefore, within the framework of the ONE-FLOW research project [26], we targeted the integrated synthesis of valsartan precursor 13 in a continuous manner over three steps (Scheme 3). Firstly, N-acylation of boronic acid pinacol ester 11 with valeryl chloride yields the functionalized intermediate 12. Secondly, Suzuki-Miyaura cross-coupling of 12 with 2-halobenzonitrile 7b-c gives biphenyl compound 5. The key step of the reaction cascade is catalyzed by heterogeneous Pd-Ce-Sn-oxides with the molecular formula Ce0.99-xSnxPd0.01O2-δ [27]. Thirdly, hydrolysis of the methyl ester group is achieved by aqueous base and yields the target compound 13. The valsartan precursor 13 can then be transformed to the targeted API 1 by the use of azides [28], but this transformation was not included in this work. However, it will be addressed in future approaches.
For the Suzuki-Miyaura cross-coupling, the use of in-house developed Pd-catalysts with the formula Ce0.99-xSnxPd0.01O2-δ is envisaged. Their applicability for cross-couplings of various ortho- and para-substituted bromoarenes with phenylboronic acid has already been reported by our group [27, 29]. The heterogeneous catalysts also proved to exhibit high functional group tolerance as well as minimal leaching behavior [27, 29]. Apart from that, the performance of continuous Suzuki cross-coupling reactions was successfully achieved by implementation of the catalysts in an in-house developed packed-bed reactor [30], referred to as Plug & Play reactor. The customized reactor features exchangeable HPLC-columns filled with catalyst particles as well as modules for heating, cooling and mixing. Obtained results [27, 29, 30] clearly indicate the high potential of the Pd-Ce-Sn-oxides for the synthesis of pharmaceutical and fine chemical intermediates in continuous flow and are promising in terms of the targeted synthesis of valsartan intermediate 13.
Results and discussion
Optimization of the individual steps in batch
In order to be able to design a continuous setup for the multistep synthesis of valsartan precursor 13, we first examined the three individual steps in batch. Since we wanted to keep the consumption of our intermediates 5, 11 and 12 to a minimum and the optimization in flow would have required a higher amount of reagents (due to the equilibration times of our targeted continuous setups), we decided to identify a first set of suitable reaction parameters in batch. However, in view of the future improvement of the process, optimization will be performed in a continuous fashion in order to take the intrinsic dynamics and all other advantages of continuous systems into account. As the pivotal step of the reaction cascade is a Suzuki-Miyaura cross-coupling, we focused on step 2 in the beginning. For this transformation we had a number of heterogeneous Pd-catalysts in hand [27], which can readily be implemented in a fixed-bed reactor for continuous flow applications [30]. Based on earlier studies, we chose to test three of them (Ce0.99-xSnxPd0.01O2-δ, x = 0.20, 0.79, 0.99) as they gave the best results in the Suzuki coupling of 2-bromobenzonitrile 7b with phenylboronic acid (standard reaction conditions: 1 mol eq. aryl halide, 1.5 mol eq. phenylboronic acid, 1.5 mol eq. K2CO3, EtOH:H2O = 7:3, 75 °C) [27]. Our initial approaches for the synthesis of valsartan 1 involved the Suzuki coupling of boronic acid derivatives carrying a (trityl-protected) tetrazole or a nitrile group in ortho-position. These attempts failed due to deactivation of the employed Pd-catalyst and substrate degradation by rapid protolytic deboronation. However, we observed that boronic acid ester 12 couples smoothly with 2-halobenzonitrile 7b-c in EtOH:H2O = 7:3 at 75 °C (Scheme 3, step 2). In the employed aqueous reaction environment 12 is hydrolyzed to the free boronic acid, which is more reactive in Suzuki-Miyaura cross-couplings than its ester analogue [31]. Therefore, we chose to pursue a synthetic route involving the Suzuki coupling of 12 with 7b-c leading to formation of valsartan precursor 13 as final product (Scheme 3). After having identified suitable cross-coupling partners, we wanted to determine the most active Pd-catalyst for our targeted transformation. Regarding the Suzuki coupling of 2-bromobenzonitrile 7b with boronic acid ester 12 using 1 mol% of catalyst, the activity follows the order Ce0.20Sn0.79Pd0.01O2-δ > Sn0.99Pd0.01O2-δ > Ce0.79Sn0.20Pd0.01O2-δ (Table 1, Entries 1–3). Hence, we decided to use Ce0.20Sn0.79Pd0.01O2-δ for all further experiments. Moreover, it is commonly known that the reactivity of aryl halides in Suzuki coupling increases along the halogen group (Cl < <Br < I). As expected, a higher conversion was obtained when using 2-iodobenzonitrile 7c instead of 7b (Table 1, Entry 4). Consequently, 7c was the coupling partner of choice and the catalyst loading was decreased from 1 mol% to 0.25 mol% for further studies.
In view of the performance of a multistep reaction cascade and the realization of N-acylation prior to the Suzuki coupling, we next needed to identify a suitable solvent system for achieving both steps in a sequential fashion. As reported in literature [32] and experienced in our lab, water is crucial for the success of the Suzuki coupling using our class of catalysts. However, step 1 needs to be performed in an aprotic organic solvent to avoid hydrolysis of the valeryl chloride reagent. Since our chosen catalyst showed no or very low activity in two-phasic solvent systems (water + dicholoromethane/toluene/ethyl acetate), we needed a single liquid phase for the Suzuki coupling step. Highly polar water-miscible organic solvents (acetonitrile; N,N-dimethyl sulfoxide; N,N-dimethylformamide) did not prove to be compatible with our catalyst. However, considerable catalyst activity was detected in aqueous ethereal solvents (tetrahydrofuran (THF); 1,4-dioxane). As THF and water are not miscible in a 1:1 ratio at elevated temperatures [33], in this case the addition of an alcoholic solvent was further necessary to obtain one liquid phase. The choice of organic solvent also has a substantial influence on the leaching behavior of palladium, tin and cerium into the reaction solution. Our investigations in this respect showed that palladium leaching increases along with the polarity of the reaction solvent (Table 2). Highest levels of palladium were determined in ethanol-water (3.12 mg kg−1), whereas they were found to be lowest in dioxane-water (0.37 mg kg−1). Cerium leaching shows the exact opposite trend (0.89 mg kg−1 in ethanol-water, 2.53 mg kg−1 in dioxane-water, Table 2). In contrast, leaching of tin was found to be insignificant in all tested solvents (0.06–0.08 mg kg−1, Table 2). Due to the fact that minimal leaching of the active catalytic species palladium was targeted, we chose dioxane-water as the solvent for the Suzuki coupling step.
Regarding the optimal dioxane-to-water ratio for the Suzuki coupling step, solubility issues leave a narrow margin. If the reaction mixture is rich in water (≥ 55 v%), employed organic compounds are not entirely soluble. If, on the other hand, dioxane is present in ≥70 v%, the inorganic base potassium carbonate (K2CO3) does not dissolve completely. An equivolume mixture of dioxane and water provided the best results for our reaction (Table 1, Entries 5–7). Apart from that, as expected the use of a larger excess of boronic acid ester 12 leads to an increased reaction rate (Table 1, Entries 8–9). Nevertheless, we decided to use 1.10 mol equivalents of 12 for the continuous reaction setup considering atom economy as well as by-product formation. Particularly, homocoupling and oxidation of the organoboron reagent were reported to occur upon depletion of the aryl halide coupling partner [27], causing a more complex reaction mixture.
After having identified optimum conditions in batch for the Suzuki coupling step, we concentrated our attention on step 1 of the reaction cascade (Scheme 3, step 1). N-acylation of 11, which was synthesized using a literature procedure [20], with valeryl chloride was performed in dioxane in presence of an organic base. We aimed for quantitative conversion of boronic acid ester 11 as previous experiments showed literature-known [34] deactivation of our Pd-catalyst, which is employed in the subsequent Suzuki coupling step, by N-H functional groups. We investigated the effect of temperature as well as molar equivalents of reagents (valeryl chloride, organic base) on the conversion (Table 3). N,N-diisopropylethylamine (DIPEA) was chosen as organic base because the formed hydrochloride salt proved to be soluble in dioxane at elevated temperatures. The rate of N-acylation of 11 increases with higher temperature (Table 3, Entries 1–3) as well as a larger excess of valeryl chloride (Table 3, Entries 4–5). Using equimolar amounts of valeryl chloride and DIPEA further accelerates the reaction (Table 3, Entry 6), achieving almost full conversion after 5 min (98.8%).
The final step of our targeted reaction cascade is the saponification of 5 to yield valsartan precursor 13 (Scheme 3, step 3). As a dioxane-water mixture was found to be optimal for the preceding Suzuki coupling step, ester hydrolysis of 5 was performed in the same solvents. At a temperature of 80 °C, the rate of methyl ester hydrolysis was found to increase with a larger molar excess of sodium hydroxide (Table 4, Entries 1–4). Substitution of the base by potassium hydroxide did not have a considerable impact on the conversion (Table 4, Entry 5). Raising the water content in the reaction solution to 55 v% slightly decelerated the conversion (Table 4, Entry 6) and a further increase to 60 v% caused solubility issues.
Optimization of sequential steps 1 and 2 in batch
For Pd-catalyzed Suzuki-Miyaura cross-couplings, the presence of a base is essential for the reaction to occur [35]. Hence, the pH of the reaction environment affects the rate of C-C bond formation [36]. In view of the performance of the Suzuki coupling subsequently to step 1, different influencing factors on the pH of the reaction mixture have to be considered. These include the formation of hydrogen chloride during the N-acylation step and hydrolysis of the excess acid chloride to the corresponding carboxylic acid upon addition of water. Consequently, the pH of the reaction mixture after step 1 is acidic and part of potassium carbonate, which is added for the Suzuki coupling, gets neutralized. In order to study the effect of the pH on the Suzuki coupling step and to identify the necessary amount of potassium carbonate to be added, we performed steps 1 and 2 in a sequential fashion in one-pot (Scheme 4).
For the sequential performance of steps 1 and 2, N-acylation of 11 was accomplished using 2 mol eq. of both valeryl chloride and DIPEA in dioxane at 80 °C. Upon completion of the reaction, the reaction mixture was quenched with an equal volume of water. Then, potassium carbonate, 2-iodobenzonitrile 7c and Pd-catalyst Ce0.20Sn0.79Pd0.01O2-δ were added to the reaction flask in order to achieve formation of 5. We compared the Suzuki coupling step performed in one-pot with the optimized separate Suzuki coupling, exhibiting a pH of 11.3 in the presence of 1.5 mol eq. of potassium carbonate (Table 5, Entry 1). When the same amount of base was used in the one-pot experiment, a pH of only 9.7 was obtained and the conversion in the Suzuki coupling step was considerably lower (Table 5, Entry 2). Therefore, another 0.17 mmol of K2CO3 were added, corresponding to the employed amount of valeryl chloride reagent in step 1. In doing so, the pH approaches the reference value and conversion was enhanced significantly (Table 5, Entry 3). Further increasing the amount of base in the reaction mixture gave comparable conversion (Table 5, Entry 4). Upon addition of 0.39 mmol of base, formation of a second liquid phase was observed (Table 5, Entry 5), which was confirmed by following the reaction with the Crystalline (Technobis) [37]. The device is designed for studying crystallization processes by turbidity measurements but also allows real time monitoring of reactions via a particle view camera. Regarding obtained results and in view of the sequential performance of steps 1 and 2 in the multistep continuous setup for the synthesis of 13, the use of 3.7 mol eq. potassium carbonate in the Suzuki coupling step was considered as most appropriate.
Continuous setups for the individual steps
After optimization of the individual steps in batch, we first targeted the realization of N-acylation as well as saponification in continuous flow. As both transformations exhibit a monophasic reaction solution, we decided to utilize a stainless steel coil (L x O.D. x I.D. 3.0 m × 1/16 in. × 0.030 in., V = 1.368 mL) as reactor in combination with a split-and-recombine unit (V = 0.565 mL) as static mixer (Scheme 5). The mixing efficiency of the latter and its applicability for rapid chemical transformations has been demonstrated lately for the aerobic oxidation of Grignard reagents in continuous flow [38]. The reactor comprises a precooling section, after which the two reagent streams are merged and introduced into a split-and-recombine section for enhanced mixing.
Regarding the continuous performance of steps 1 and 3, a dual syringe infusion pump (LA-120, Landgraf) was utilized for steady reagent delivery into the reactor setup (V = 1.933 mL), which was assembled using standard HPLC fittings. The performance of the continuous setup at ambient pressure was determined at three different total flow rates based on the conversion of 11 and 5, respectively (for details see Supporting Information). In both cases, substrate conversion of >99% was obtained at the lowest flow rate of 0.10 mL min−1, which corresponds to a calculated residence time of τ~19 min (Table 6, Entry 1). Whereas the N-acylation step proved to be similarly efficient at higher flow rates of 0.15 and 0.20 mL min−1, for methyl ester hydrolysis conversion decreased noticeably (Table 6, Entries 2–3). However, the observed general trend of higher conversion at lower flow rates suggests that the mixing obtained with the split-and-recombine reactor is sufficient for both transformations.
Next, we focused on the continuous setup for the second step of our cascade towards 13, Suzuki-Miyaura cross-coupling. The applicability of our heterogeneous Pd-catalyst for the continuous Suzuki coupling of various substituted bromoarenes with phenylboronic utilizing the Plug & Play reactor has already been reported by our group [29, 30]. Therefore, we decided to adopt this approach for the C-C cross-coupling of boronic acid ester 12 with aryl halide 7c. In view of the performance of our three-step reaction cascade, we based the experiment design on the preceding N-acylation step. For step 1 quantitative conversion of 11 is targeted, which is achieved in dioxane using a flow rate of 0.10 mL min−1. As we identified an equivolume dioxane-water mixture as optimal for subsequent Suzuki coupling, we intended to merge the reagent stream of step 1 with an aqueous stream of the same flow rate. Consequently, for testing step 2 separately in a continuous fashion, we chose a flow rate of 0.20 mL min−1. In the experiment, a pre-mixed reagent solution containing both coupling partners as well as potassium carbonate was pumped through the Plug & Play reactor [30], which was equipped with an HPLC column (L x I. D. 120 × 8 mm) filled with catalyst particles (4.7 g) (Scheme 6, for details see Supporting Information). Due to the backpressure of the catalyst bed (2–3 bar) and incompatibility of the solvent mixture with the available HPLC pump, a high-pressure syringe pump (VIT-FIT HP, LAMBDA Instruments) in combination with stainless steel syringes was utilized. At the chosen flow rate of 0.2 mL min−1, the mean residence time inside the HPLC column containing the catalytic species was determined by measurement of a tracer-determined residence time distribution curve to be 22.3 min. Utilizing the abovementioned setup, step 2 of the targeted reaction cascade was successfully performed in a continuous fashion. After an initial equilibration phase of 50 min (consuming 10 mL of stock solution), the target compound 5 was obtained with an average 95% yield (Fig. 1).
Regarding the performance of Suzuki cross-coupling reactions in continuous flow, leaching of catalytically active Pd species from the solid support into the reaction solution is known to be a major issue [39, 40]. Therefore, the contents of palladium, cerium and tin in the outlet flow of the continuous experiment were measured by ICP-MS after certain time points (Table 7). As already observed in the batch experiments, the amount of tin in the reaction solution was found to be negligible in all samples (<0.02 mg kg−1). The concentration of cerium in the outlet flow instead was determined to decrease over the course of the continuous experiment and was quantified to be 0.70 mg kg−1 after 4 h. As already reported previously [29], the heterogeneous catalysts with the molecular formula Ce0.99-xSnxPd0.01O2-δ show a significant loss of palladium during the initial phase (0–120 min) of the Suzuki cross-coupling in continuous flow (literature conditions: 1.10 g of Pd-catalyst, flow rate 0.45 mL min−1). However, after equilibration of the system the palladium content drops below the limit of quantification [29]. Likewise, considerable leaching of palladium into the reaction solution was also observed in the continuous Suzuki coupling step for the synthesis of valsartan precursor 5 over the entire runtime of the experiment. Presumably, due to the lower flow rate of 0.2 mL min−1 and the larger amount of catalyst compared to mentioned literature experiment [29], the applied system has not reached its steady-state within the duration of the experiment, which was limited by the availability of boronic acid ester 12. However, in view of a possible application of the reaction setup for continuous Suzuki coupling on a larger scale, the implementation of a palladium scavenging strategy is advisable. Galaffu et al. reported the successful use of different sulfur-based silica scavengers for the removal of palladium from a variety of advanced synthetic intermediates including valsartan precursor 5 [19]. In their study, after the Suzuki coupling step (1 mol% Pd(PPh3)4, DME/EtOH/H2O, Na2CO3) the residual amount of palladium in product 5 was effectively decreased from 2100 ppm to <1 ppm by the utilization of respective functionalized silica frameworks [19].
Multistep continuous setup for the synthesis of 13
After confirming the feasibility of the three single steps of the reaction cascade in continuous flow, an integrated setup for their consecutive performance was developed by simple combination of the individual reaction modules (Scheme 7). The N-acylation step was performed in the coil reactor setup already described using a flow rate of 0.10 mL min−1 by means of high-pressure syringe pumps (VIT-FIT HP, Lambda Instruments). The outlet stream was then quenched with an aqueous potassium carbonate solution delivered by an HPLC pump (0.10 mL min−1, P4.1S, Knauer) using a T-mixing element. For the key step of the reaction cascade, Suzuki-Miyaura cross-coupling, the reaction solution was introduced at resulting 0.20 mL min−1 into the Plug & Play reactor [30] equipped with a fixed-bed of heterogeneous Pd-catalyst Ce0.20Sn0.79Pd0.01O2-δ. Finally, the process stream was merged with a sodium hydroxide solution supplied by a syringe pump (0.1 mL min−1, LA-120, Landgraf) to achieve methyl ester hydrolysis. For this step, a longer reactor coil (PTFE, L x O.D. x I.D. 10.0 m × 1/16 in. × 0.031 in.) was chosen in comparison to the individual setup in order to compensate for the higher flow rate of 0.3 mL min−1 and to achieve a similar residence time. With this setup exhibiting an estimated total residence time of roughly 1 h, the three steps of the reaction cascade were successfully performed continuously in a consecutive fashion for over 6 h. After an initial equilibration phase (260 min), quantitative conversion of 2-iodobenzonitrile 7c was achieved and the target compound 13 was obtained with up to 96% yield (90 ± 4% yield, values fluctuating around the mean) and 73% enantiomeric excess (determined by achiral and chiral HPLC, respectively; see Supporting Information). Performing the analogous three-step reaction sequence from 11 to 13 in batch including purification of intermediates 5 and 12, the obtained overall yield was only 28% (see Supporting Information for the performance of the individual steps in batch). Therefore, translation of the multistep cascade from batch to continuous flow allowed a significant increase in yield of valsartan precursor 13. Addressing the moderate enantiopurity of compound 13 synthesized in continuous flow, racemization apparently occurred during the saponification step as intermediates 5 and 12 were shown to be enantiopure (see Supporting information for determination of enantiopurity of 5 and 12). Consequently, in view of a potential application of the developed continuous approach for actual API production, further process optimization is required to achieve the synthesis of an enantiomerically pure compound.
Regarding the selectivity of the reaction steps, in Ce0.99-xSnxPd0.01O2-δ catalyzed Suzuki cross-coupling oxidation and homocoupling of the boronic acid species were reported to cause minor side product formation upon depletion of the aryl halide coupling partner [27]. Therefore, only a slight excess of 1.10 mol eq. of boronic acid ester 12 was employed to minimize formation of the respective by-products, which were presumably present in the reaction mixture and can be removed by chromatographic techniques. Apart from that, N-acylation as well as methyl ester hydrolysis proved to be rather clean reaction steps. This is supported by the fact that HPLC analysis of the outlet flow of the thee-step continuous process revealed side products to the extent of 9 area% compared to the product peak (λ = 230 nm), which most likely originate from the 10% excess of 12.
Conclusion
In conclusion, we developed an approach for the integrated multistep synthesis of a late-stage precursor of the active pharmaceutical ingredient valsartan using a modular continuous setup composed of two coil reactor units and a fixed-bed reactor. After equilibration of the multistep setup, it was demonstrated to successfully yield the target compound 13 with up to 96% yield with a moderate ee. Whereas in published approaches for the continuous synthesis of a valsartan precursor in a packed-bed reactor little functionalized biphenyls were obtained [21, 22], with our described setup we were able to perform the synthesis of a more advanced valsartan precursor, in this way highlighting the broad functional-group tolerance of the employed heterogeneous palladium catalyst. However, leaching of palladium into the reaction solution was observed for the heterogeneous catalyst employed in the Suzuki-Miyaura cross-coupling step. Hence, there is room for improvement of the process, for example by implementation of a Pd scavenger or by utilization of a more leaching-resistant Pd catalyst. Nevertheless, with the development of the three-step continuous setup, the applicability of continuous flow techniques for the synthesis of advanced chemical intermediates has been proven once more and hopefully encourages research in this field.
Experimental section
General information
Chemicals and solvents were purchased from commercial suppliers and used as received [Ark Pharm: 2-(4-(bromomethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (97%); Fluorochem: l-valine methyl ester hydrochloride (99%), 2-iodobenzonitrile (98%); Sigma Aldrich: potassium carbonate (99%), valeryl chloride (98%), anisole (99%); Carl Roth: dioxane (>99.5%), NaOH (>99%)]. Analytical thin layer chromatography was performed on pre-coated aluminium plates (Merck, silica gel 60, F254) and spots were visualized with UV light (254 nm) and potassium permanganate stain. Column chromatography purifications were carried out using MN silica gel 60 (70–230 mesh). For monitoring of the reaction progress and determination of enantiomeric excess, an Agilent 1100 series HPLC system was utilized (for HPLC parameters see Supporting Information). HPLC-MS measurements were performed on a Waters Acquity H-Class system equipped with a Waters Acquity SQD detector. For ICP-MS measurements, an Agilent 7700x ICP-MS system was employed after microwave assisted acidic digestion of the samples. Measurement of the pH was achieved using a Mettler Toledo FiveEasy™ pH bench meter equipped with a Mettler Toledo LE409 probe. NMR-measurements were recorded using a Bruker Avance III 300 MHz spectrometer (1H: 300 MHz, 13C: 75 MHz, for NMR-data see Supporting Information).
Batch experiments for optimization of step 1
In a typical N-acylation experiment, borate 11 (82.5 μmol, 55 mM), DIPEA (1.05–2.00 mol eq.) and anisole (133 mM, internal standard) were dissolved in dioxane to achieve a total volume of 1.5 mL. To start the reaction, valeryl chloride (1.05–2.00 mol eq.) was added at room temperature and the reaction was kept stirring (2000 rpm) with a magnetic stirrer at elevated temperature (40–80 °C) for 20 min. To examine the reaction progress, an aliquot (20 μL) was withdrawn from the reaction solution, quenched with MeOH:H3PO4 = 50:50 (400 μL) and analyzed by HPLC (Method A, see Supporting Information for details).
Batch experiments for optimization of step 2
For optimization of Suzuki-Miyaura cross-coupling reaction, 2-halobenzonitrile 7b-c (75 μmol–150 μmol, 25 mM), borate 12 (1.1–1.5 mol eq.), K2CO3 (1.5 mol eq.) and anisole (67 mM, internal standard) were dissolved in the respective reaction solvent to achieve a total volume of 3–6 mL. Then, Ce0.99-xSnxPd0.01O2-δ (2.1–21.4 mg, corresponding to 0.05–1 mol% Pd) was added and the reaction mixture was stirred (2000 rpm) at elevated temperature (65–85 °C) for 90–120 min. For investigation of the reaction progress, an aliquot (40 μL) was withdrawn from the reaction solution, quenched with MeOH:H3PO4 buffer = 55:45 (400 μL) and analyzed by HPLC (Method B for reactions with 7c, method C for reactions with 7b, see Supporting Information for details). For determination of leaching of Pd, Ce and Sn, the reaction solution was filtered, the filter washed with the reaction solvent and the solvent was evaporated. Obtained solid residue was then analyzed by ICP-MS after microwave assisted acidic digestion of the samples.
Batch experiments for optimization of step 3
In a typical methyl ester hydrolysis experiment, biphenyl compound 5 (60 μmol, 20 mM) and anisole (67 mM, internal standard) were dissolved in the respective reaction solvent to achieve a total volume of 2.95 mL. To initiate the reaction, aqueous base (NaOH or KOH, 50 μL, 2.5–20 mol eq.) was added and the reaction mixture was stirred (2000 rpm) at 80 °C for 30 min. To monitor the reaction progress, an aliquot (40 μL) was quenched with MeOH:H3PO4 buffer = 55:45 (400 μL) and analyzed by HPLC (Method B, see Supporting Information for details).
Batch experiments for optimization of sequential steps 1 and 2
For optimization of the sequential performance of steps 1 and 2 in batch, borate 11 (82.5 μmol, 55 mM), DIPEA (2.00 mol eq.) and anisole (185 mM, internal standard) were dissolved in dioxane to achieve a total volume of 1.5 mL. To start the reaction, valeryl chloride (2.00 mol eq.) was added at room temperature and the reaction was kept stirring (2000 rpm) at 80 °C. After 20 min of reaction, deionized water (1.5 mL), potassium carbonate (0.11–0.50 mmol) and 2-iodobenzonitrile 7c (75 μmol) were added to the reaction solution. Then Ce0.20Sn0.79Pd0.01O2-δ (2.7 mg, corresponding to 0.25 mol% Pd) was added and the reaction mixture was stirred (2000 rpm) at 80 °C for 60 min. For investigation of the reaction progress, an aliquot (40 μL) was withdrawn from the reaction solution, quenched with MeOH:H3PO4 buffer = 55:45 (400 μL) and analyzed by HPLC (Method B, see Supporting Information for details).
Integrated synthesis of 13 in continuous flow
Solution A [boronic acid ester 11 (110 mM, 1.1 mol eq.), 2-iodobenzonitrile 7c (100 mM), DIPEA (220 mM, 2.2 mol eq.), anisole (240 mM) as internal standard] and solution B [valeryl chloride (220 mM, 2.2 mol eq.)] were prepared in dioxane and degassed in an ultrasonic bath for 15 min. After equilibration of the reactor system, the two reagent solutions were introduced into the first part of the reactor for N-acylation (step 1, split-and-recombine reactor +3 m PEEK coil L x O.D. x I.D. 10.0 m × 1/16 in. × 0.030 in., Vtotal = 1.93 mL, τ = 19.3 min) by means of high-pressure syringe pumps (VIT-FIT HP, Lambda Instruments) at 80 °C using a flow rate of 0.05 mL min−1 (resulting in a total flow rate of 0.1 mL min−1 for step 1). The outlet stream was quenched with an aqueous solution of potassium carbonate (185 mM, 3.7 mol eq.) delivered by an HPLC pump (P4.1S, Knauer) with a flow rate of 0.1 mL min−1 using a T-mixing element. The resulting reaction solution was then pumped through the Plug & Play reactor [30] equipped with a preparative HPLC column (L x I.D. 120 × 8 mm) filled with Pd-catalyst Ce0.20Sn0.79Pd0.01O2-δ (4.3 g) for Suzuki-Miyaura cross-coupling (τ = 22.3 min). Subsequently, the process stream was merged with a sodium hydroxide solution (750 mM, 15 mol eq., dioxane:H2O = 2:3) with a flow rate of 0.1 mL min−1 delivered by a syringe pump (LA-120, Landgraf) and introduced into the final part of the reactor to achieve methyl ester hydrolysis (step 3, split-and-recombine reactor +10 m PEEK coil L x O.D. x I.D. 10.0 m × 1/16 in. × 0.030 in., Vtotal = 5.13 mL, τ = 17.1 min) to obtain targeted valsartan precursor 13. After certain time points, an aliquot of the outlet flow (50 μL) was quenched with MeOH:H3PO4 = 55:45 (400 μL) and analyzed by HPLC (Method B).
References
Cardiovascular diseases (2017) World Health Organization. http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 4 Sept 2018
Oparil S, Schmieder RE (2015). Circ Res 116:1074–1095
Goossen LJ, Melzer B (2007). J Org Chem 72:7473–7476
Kloss F, Neuwirth T, Haensch VG, Hertweck C (2018). Angew Chem Int Ed Engl 57:14476–14481
Siddiqui N, Husain A, Chaudhry L, Alam MS, Mitra M, Bhasi PS (2011). J Appl Pharm Sci 01:12–19
Li JJ, Corey EJ (2013) Drug discovery: practices, processes, and perspectives. Wiley-VCH, New Jersey
Davis J, Oparil S (2018). Curr Hypertens Rep 20:90
Vilela-Martin JF (2016). Drug Des, Dev Ther 10:1627–1639
Bühlmayer P, Ostermayer F, Schmidlin T (1991) Acyl compounds. Eur Patent EP 1991–810098, Aug 28, 1991; (1992) Chem Abstr 116:151772
Lamberth C, Dinges J (2016) Bioactive Carboxylic Compound Classes. Wiley-VCH, New Jersey; Chapter 7, p 91
Verardo G, Geatti P, Castaldi G, Toniutti N, Allegrini P (2005) A process for the preparation of valsartan and intermediates thereof. Eur patent application EP 1 533 305 A1, mar 25, 2005; (2005). Chem Abstr 142:482034
Sedelmeier G (2013) Process for the preparation of tetrazole derivatives from organo boron and organo aluminium azides. US patent US 8,569,528 B2, Oct 29, 2013; (2016). Chem Abstr 166:362689
Villa M, Allegrini P, Arrighi K, Paiocchi M (2001) Ortho-metalation process for the synthesis of 2-substituted-1-(tetrazol-5-yl)benzenes. US patent US 6,271,375 B1, Aug 7, 2001; (1999). Chem Abstr 130:125215
Zhang CX, Zheng GJ, Bi FQ, Li YL (2008). Chin Chem Lett 19:759–761
Zhang C, Zheng G, Fang L, Li Y (2006). Synlett 03:475–477
Tsiperman E, Fine S, Yurkovsky S, Braude V (2007) Process for preparing valsartan. PCT Int Appl WO 2007/005967 A2, Jan 11, 2007; (2007). Chem Abstr 146:122299
Seki M, Nagahama M (2011). J Org Chem 76:10198–10206
Ghosh S, Kumar AS, Mehta GN (2010). Beilstein J Org Chem 6:4–7
Galaffu N, Man SP, Wilkes RD, Wilson JRH (2007). Org Process Res Dev 11:406–413
Bessa Belmunt J, Huguet Clotet J, Perez Andres JA, Dalmases Barjoan P (2005) Process for the preparation of valsartan and precursors thereof. PCT Int Appl WO 2005/102987 A1, Nov 3, 2005; (2005). Chem Abstr 143:406147
Pandarus V, Gingras G, Béland F, Ciriminna R, Pagliaro M (2014). Org Process Res Dev 18:1550–1555
Nagaki A, Hirose K, Tonomura O, Taniguchi S, Taga T, Hasebe S, Ishizuka N, Yoshida J-i (2016). Org Process Res Dev 20:687–691
Newman SG, Jensen KF (2013). Green Chem 15:1456–1472
FDA perspective on continuous manufacturing (2012) US food and drug Administation. https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM341197.pdf. Accessed 21 Jan 2019
Statement from FDA (2018) US Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm619024.htm. Accessed 30 Oct 2018
ONE-FLOW Research Project Home Page. https://one-flow.org. Accessed 20 Feb 2019
Lichtenegger GJ, Maier M, Hackl M, Khinast JG, Gössler W, Griesser T, Kumar VSP, Gruber-Woelfler H, Deshpande PA (2017). J Mol Catal A Chem 426:39–51
Roh J, Vávrová K, Hrabálek A (2012). Eur J Org Chem 2012:6101–6118
Lichtenegger GJ, Maier M, Khinast JG, Gruber-Wölfler H (2016). J Flow Chem 6:244–251
Lichtenegger GJ, Tursic V, Kitzler H, Obermaier K, Khinast JG, Gruber-Wölfler H (2016). Chem Ing Tech 88:1518–1523
Huang F, Yip H-L, Cao Y (2016) Polymer photovoltaics: materials, physics, and dsevice engineering. Royal Society of Chemistry, Cambridge
Smith MD, Stepan AF, Ramarao C, Brennan PE, Ley SV (2003). Chem Commun 3:2652–2653
Smith MD, Mostofian B, Petridis L, Cheng X, Smith JC (2016). J Phys Chem B 120:740–747
Duefert MA, Billingsley KL, Buchwald SL (2013). J Am Chem Soc 135:12877–12885
Suzuki A (2002). J Organomet Chem 653:83–90
Hoffmann I, Blumenröder B, Onodi neé Thumann S, Dommer S, Schatz J (2015). Green Chem 17:3844–3857
Technobis Crystallization Systems Homepage. https://www.crystallizationsystems.com/Crystalline. Accessed 8 May 2019
Maier MC, Lebl R, Sulzer P, Lechner J, Mayr T, Zadravec M, Slama E, Pfanner S, Schmölzer C, Pöchlauer P, Kappe CO, Gruber-Woelfler H (2019). React Chem Eng 4:393–401
Bourouina A, Meille V, de Bellefon C (2018). J Flow Chem 8:117–121
Barreiro EM, Hao Z, Adrio LA, van Ommen JR, Hellgardt K, Hii KK (2018). Catal Today 308:64–70
Acknowledgments
Open access funding provided by Graz University of Technology. The authors kindly acknowledge the funding by the H2020FETOPEN-2016-2017 program of the European Commission (Grant agreement number: 737266-ONE FLOW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article Highlights
• Development of a three-step synthesis of an advanced valsartan precursor in continuous flow.
• Consecutive continuous performance of N-acylation, Suzuki-Miyaura coupling and methyl ester hydrolysis.
• Combination of coil reactors and a fixed-bed reactor for the continuous reaction setup.
Electronic supplementary material
ESM 1
(PDF 1604 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hiebler, K., Soritz, S., Gavric, K. et al. Multistep synthesis of a valsartan precursor in continuous flow. J Flow Chem 10, 283–294 (2020). https://doi.org/10.1007/s41981-019-00044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00044-x