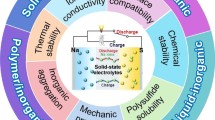
Abstract
Because sodium-ion batteries are relatively inexpensive, they have gained significant traction as large-scale energy storage devices instead of lithium-ion batteries in recent years. However, sodium-ion batteries have a lower energy density than lithium-ion batteries because sodium-ion batteries have not been as well developed as lithium-ion batteries. Solid-state batteries using solid electrolytes have a higher energy density than liquid batteries in regard to applications with sodium-ion batteries, making them more suitable for energy storage systems than liquid batteries. Due to their low ionic conductivity, solid electrolytes are currently unable to achieve comparable performance to liquid electrolytes at room temperature. In this review, we discuss the advancements in SSEs applied to sodium-ion batteries in recent years, including inorganic solid electrolytes, such as Na–β-Al2O3, NASICON and Na3PS4, polymer solid electrolytes based on PEO, PVDF-HFP and PAN, and plastic crystal solid electrolytes mainly composed of succinonitrile. Additionally, appropriate solutions for low ionic conductivity, a narrow electrochemical stability window and poor contact with electrodes, which are the significant flaws in current SSEs, are discussed in this review.
Graphical Abstract



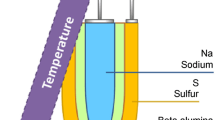
Copyright © 2017, Royal Society of Chemistry; b ionic conductivity of SSEs and liquid electrolytes at different temperatures. Reprinted with permission from Ref. [55]. Copyright © 2018, John Wiley and Sons


Copyright © 2018, Elsevier

Copyright © 2020, Elsevier

Copyright © 2020, American Chemical Society

Copyright © 2021, John Wiley and Sons

Copyright © 2019, John Wiley and Sons

Copyright © 2021, John Wiley and Sons

Copyright © 2016, John Wiley and Sons

Copyright © 2018, American Chemical Society

Copyright © 2019, John Wiley and Sons

Copyright © 2016, John Wiley and Sons

Copyright © 2020, American Chemical Society

Copyright © 2019, Elsevier

Copyright © 2022, Royal Society of Chemistry


Copyright © 2015, Royal Society of Chemistry

Copyright © 2021, Springer

Copyright © 2019, American Chemical Society

Copyright © 2020, Springer-Verlag GmbH Germany, part of Springer Nature

Copyright © 2022, American Chemical Society

Copyright © 2020, Elsevier

Copyright © 2016, Royal Society of Chemistry


Copyright © 2022, John Wiley and Sons

Copyright © 2021, Taylor & Francis

Copyright © 2022, John Wiley and Sons

Copyright © 2021, American Chemical Society

Copyright © 2021, American Chemical Society

Copyright © 2021 The Author(s)

Copyright © 2021, Elsevier

Copyright © 2022, American Chemical Society

Copyright © 2021, Springer Nature

Copyright © 2020, Elsevier

Copyright © 2022, John Wiley and Sons

Copyright © 2022, American Chemical Society


Copyright © 2015 Elsevier Ltd. All rights reserved

Copyright © 2019 Elsevier Ltd. All rights reserved

Copyright © 2021, American Chemical Society


Similar content being viewed by others
References
Zhou, C.T., Bag, S., Thangadurai, V.: Engineering materials for progressive all-solid-state Na batteries. ACS Energy Lett. 3, 2181–2198 (2018). https://doi.org/10.1021/acsenergylett.8b00948
Peng, L.S., Wei, Z.D.: Catalyst engineering for electrochemical energy conversion from water to water: water electrolysis and the hydrogen fuel cell. Engineering 6, 653–679 (2020). https://doi.org/10.1016/j.eng.2019.07.028
Wang, Z.Y., Zhao, Z.J., Baucom, J., et al.: Nitrogen-doped graphene foam as a metal-free catalyst for reduction reactions under a high gravity field. Engineering 6, 680–687 (2020). https://doi.org/10.1016/j.eng.2019.12.018
Cai, Y., Chu, G.W., Luo, Y., et al.: An evaluation of metronidazole degradation in a plasma-assisted rotating disk reactor coupled with TiO2 in aqueous solution. Engineering 7, 1603–1610 (2021). https://doi.org/10.1016/j.eng.2020.03.020
Huang, X.J., Qb, M., Chen, H.J., et al.: Renewable energy conversion, storage, and efficient utilization. Science 360, 47–51 (2018)
Du, S.F.: Recent advances in electrode design based on one-dimensional nanostructure arrays for proton exchange membrane fuel cell applications. Engineering 7, 33–49 (2021). https://doi.org/10.1016/j.eng.2020.09.014
Mohideen, M.M., Radhamani, A.V., Ramakrishna, S., et al.: Recent insights on iron based nanostructured electrocatalyst and current status of proton exchange membrane fuel cell for sustainable transport. J. Energy Chem. 69, 466–489 (2022). https://doi.org/10.1016/j.jechem.2022.01.035
Marappan, M., Palaniswamy, K., Velumani, T., et al.: Performance studies of proton exchange membrane fuel cells with different flow field designs-review. Chem. Rec. 21, 663–714 (2021). https://doi.org/10.1002/tcr.202000138
Steele, B.C.H., Heinzel, A.: Materials for fuel-cell technologies. Nature 414, 345–352 (2001). https://doi.org/10.1038/35104620
Lim, B., Jiang, M.J., Camargo, P.H.C., et al.: Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324, 1302–1305 (2009). https://doi.org/10.1126/science.1170377
Hu, Y.S.: Batteries: getting solid. Nat. Energy 1, 16042 (2016). https://doi.org/10.1038/nenergy.2016.42
Wu, T., Wen, Z.Y., Sun, C.Z., et al.: Disordered carbon tubes based on cotton cloth for modulating interface impedance in β″-Al2O3-based solid-state sodium metal batteries. J. Mater. Chem. A 6, 12623–12629 (2018). https://doi.org/10.1039/c8ta01883a
Larcher, D., Tarascon, J.M.: Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015). https://doi.org/10.1038/nchem.2085
Goodenough, J.B.: How we made the Li-ion rechargeable battery. Nat. Electron. 1, 204 (2018). https://doi.org/10.1038/s41928-018-0048-6
Yabuuchi, N., Kubota, K., Dahbi, M., et al.: Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014). https://doi.org/10.1021/cr500192f
Service RF: Hydrogen cars: fad or the future? Science 324, 1257–1259 (2009). https://doi.org/10.1126/science.324_1257
Hwang, J.Y., Myung, S.T., Sun, Y.K.: Sodium-ion batteries: present and future. Chem. Soc. Rev. 46, 3529–3614 (2017). https://doi.org/10.1039/c6cs00776g
Che, H.Y., Chen, S.L., Xie, Y.Y., et al.: Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 10, 1075–1101 (2017). https://doi.org/10.1039/c7ee00524e
Kim, H., Kim, H., Ding, Z., et al.: Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 6, 1600943 (2016). https://doi.org/10.1002/aenm.201600943
Ramesh, A., Tripathi, A., Balaya, P.: A mini review on cathode materials for sodium-ion batteries. Int. J. Appl. Ceram. Technol. 19, 913–923 (2022). https://doi.org/10.1111/ijac.13920
Huang, Q., Chen, G.X., Zheng, P., et al.: NASICON-structured Na ion conductor for next generation energy storage. Funct. Mater. Lett. 14, 2130005 (2021). https://doi.org/10.1142/s179360472130005x
Palomares, V., Serras, P., Villaluenga, I., et al.: Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 5, 5884–5901 (2012). https://doi.org/10.1039/C2EE02781J
Deng, J.Q., Luo, W.B., Chou, S.L., et al.: Sodium-ion batteries: from academic research to practical commercialization. Adv. Energy Mater. 8, 1701428 (2018). https://doi.org/10.1002/aenm.201701428
Pan, H.L., Hu, Y.S., Chen, L.Q.: Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 6, 2338–2360 (2013). https://doi.org/10.1039/c3ee40847g
Peng, J., Zhang, W., Liu, Q.N., et al.: Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv. Mater. 34, 2108384 (2022). https://doi.org/10.1002/adma.202108384
Chae, M.S., Elias, Y., Aurbach, D.: Tunnel-type sodium manganese oxide cathodes for sodium-ion batteries. ChemElectroChem 8, 798–811 (2021). https://doi.org/10.1002/celc.202001323
Wang, Y.S., Feng, Z.M., Cui, P.X., et al.: Pillar-beam structures prevent layered cathode materials from destructive phase transitions. Nat. Commun. 12, 13 (2021). https://doi.org/10.1038/s41467-020-20169-1
Peters, J., Buchholz, D., Passerini, S., et al.: Life cycle assessment of sodium-ion batteries. Energy Environ. Sci. 9, 1744–1751 (2016). https://doi.org/10.1039/c6ee00640j
Reddy, M.V., Mauger, A., Julien, C.M., et al.: Brief history of early lithium-battery development. Materials 13, 1884 (2020). https://doi.org/10.3390/ma13081884
Zeng, Y.: Sodium metal batteries, electrochemical devices. China Patent CN114824167A, 29 Jul 2022
Tian, Y., An, Y.L., Wei, C.L., et al.: Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 78, 105344 (2020). https://doi.org/10.1016/j.nanoen.2020.105344
Nakamoto, K., Sakamoto, R., Sawada, Y., et al.: Over 2 V aqueous sodium-ion battery with Prussian blue-type electrodes. Small Methods 3, 1800220 (2019). https://doi.org/10.1002/smtd.201800220
Jeong, S., Kim, B.H., Park, Y.D., et al.: Artificially coated NaFePO4 for aqueous rechargeable sodium-ion batteries. J. Alloys Compd. 784, 720–726 (2019). https://doi.org/10.1016/j.jallcom.2019.01.046
Zhang, J., Wang, D.W., Lv, W., et al.: Ethers illume sodium-based battery chemistry: uniqueness, surprise, and challenges. Adv. Energy Mater. 8, 1801361 (2018). https://doi.org/10.1002/aenm.201801361
Mauger, Julien, Paolella, et al.: Building better batteries in the solid state: a review. Materials 12, 3892 (2019). https://doi.org/10.3390/ma12233892
Jin, T., Ji, X., Wang, P.F., et al.: High-energy aqueous sodium-ion batteries. Angew. Chem. Int. Ed. 60, 11943–11948 (2021). https://doi.org/10.1002/anie.202017167
Jiang, P., Lei, Z.Y., Chen, L., et al.: Polyethylene glycol-Na+ interface of vanadium hexacyanoferrate cathode for highly stable rechargeable aqueous sodium-ion battery. ACS Appl. Mater. Interfaces 11, 28762–28768 (2019). https://doi.org/10.1021/acsami.9b04849
Bin, D., Wang, F., Tamirat, A.G., et al.: Progress in aqueous rechargeable sodium-ion batteries. Adv. Energy Mater. 8, 1703008 (2018). https://doi.org/10.1002/aenm.201703008
Zhang, H., Qin, B.S., Han, J., et al.: Aqueous/nonaqueous hybrid electrolyte for sodium-ion batteries. ACS Energy Lett. 3, 1769–1770 (2018). https://doi.org/10.1021/acsenergylett.8b00919
Li, Q., Cao, Z., Wahyudi, W., et al.: Unraveling the new role of an ethylene carbonate solvation shell in rechargeable metal ion batteries. ACS Energy Lett. 6, 69–78 (2021). https://doi.org/10.1021/acsenergylett.0c02140
Olsson, E., Cottom, J., Alptekin, H., et al.: Investigating the role of surface roughness and defects on EC breakdown, as a precursor to SEI formation in hard carbon sodium-ion battery anodes. Small 18, 2200177 (2022). https://doi.org/10.1002/smll.202200177
Dubois, M., Ghanbaja, J., Billaud, D.: Electrochemical intercalation of sodium ions into poly(para-phenylene) in carbonate-based electrolytes. Synth. Met. 90, 127–134 (1997). https://doi.org/10.1016/S0379-6779(97)81261-1
Hofmann, A., Wang, Z.Q., Bautista, S.P., et al.: Comprehensive characterization of propylene carbonate based liquid electrolyte mixtures for sodium-ion cells. Electrochim. Acta 403, 139670 (2022). https://doi.org/10.1016/j.electacta.2021.139670
Subramanyan, K., Lee, Y.S., Aravindan, V.: Impact of carbonate-based electrolytes on the electrochemical activity of carbon-coated Na3V2(PO4)2F3 cathode in full-cell assembly with hard carbon anode. J. Colloid Interface Sci. 582, 51–59 (2021). https://doi.org/10.1016/j.jcis.2020.08.043
Kamath, G., Cutler, R.W., Deshmukh, S.A., et al.: In silico based rank-order determination and experiments on nonaqueous electrolytes for sodium ion battery applications. J. Phys. Chem. C 118, 13406–13416 (2014). https://doi.org/10.1021/jp502319p
Liu, Q., Wu, F., Mu, D.B., et al.: A theoretical study on Na+ solvation in carbonate ester and ether solvents for sodium-ion batteries. Phys. Chem. Chem. Phys. 22, 2164–2175 (2020). https://doi.org/10.1039/c9cp05636j
Ponrouch, A., Monti, D., Boschin, A., et al.: Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 3, 22–42 (2015). https://doi.org/10.1039/c4ta04428b
Wan, M., Tang, Y., Wang, L.L., et al.: Core-shell hexacyanoferrate for superior Na-ion batteries. J. Power Sources 329, 290–296 (2016). https://doi.org/10.1016/j.jpowsour.2016.08.059
Viet Thieu, Q.Q., Hoang, H., Le, V.T., et al.: Enhancing electrochemical performance of sodium Prussian blue cathodes for sodium-ion batteries via optimizing alkyl carbonate electrolytes. Ceram. Int. 47, 30164–30171 (2021). https://doi.org/10.1016/j.ceramint.2021.07.195
Jang, J.Y., Kim, H., Lee, Y., et al.: Cyclic carbonate based-electrolytes enhancing the electrochemical performance of Na4Fe3(PO4)2(P2O7) cathodes for sodium-ion batteries. Electrochem. Commun. 44, 74–77 (2014). https://doi.org/10.1016/j.elecom.2014.05.003
Ponrouch, A., Marchante, E., Courty, M., et al.: In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 5, 8572–8583 (2012). https://doi.org/10.1039/c2ee22258b
Jache, B., Adelhelm, P.: Use of graphite as a highly reversible electrode with superior cycle life for sodium-ion batteries by making use of co-intercalation phenomena. Angew. Chem. Int. Ed. 53, 10169–10173 (2014). https://doi.org/10.1002/anie.201403734
Lin, Z.H., Xia, Q.B., Wang, W.L., et al.: Recent research progresses in ether- and ester-based electrolytes for sodium-ion batteries. InfoMat 1, 376–389 (2019). https://doi.org/10.1002/inf2.12023
Slater, M.D., Kim, D., Lee, E., et al.: Sodium-ion batteries. Adv. Funct. Mater. 23, 947–958 (2013). https://doi.org/10.1002/adfm.201200691
Zhao, C.L., Liu, L.L., Qi, X.G., et al.: Solid-state sodium batteries. Adv. Energy Mater. 8, 1703012 (2018). https://doi.org/10.1002/aenm.201703012
Amaral, M.M., Venâncio, R., Peterlevitz, A.C., et al.: Recent advances on quasi-solid-state electrolytes for supercapacitors. J. Energy Chem. 67, 697–717 (2022). https://doi.org/10.1016/j.jechem.2021.11.010
Janek, J., Zeier, W.G.: A solid future for battery development. Nat. Energy 1, 16141 (2016). https://doi.org/10.1038/nenergy.2016.141
Guin, M., Tietz, F., Guillon, O.: New promising NASICON material as solid electrolyte for sodium-ion batteries: correlation between composition, crystal structure and ionic conductivity of Na3+xSc2SixP3−xO12. Solid State Ion. 293, 18–26 (2016). https://doi.org/10.1016/j.ssi.2016.06.005
Monti, D., Jónsson, E., Palacín, M.R., et al.: Ionic liquid based electrolytes for sodium-ion batteries: Na+ solvation and ionic conductivity. J. Power Sources 245, 630–636 (2014). https://doi.org/10.1016/j.jpowsour.2013.06.153
Kaur, G., Kumar, H., Singla, M.: Diverse applications of ionic liquids: a comprehensive review. J. Mol. Liq. 351, 118556 (2022). https://doi.org/10.1016/j.molliq.2022.118556
Ghandi, K.: A review of ionic liquids, their limits and applications. Green Sustain. Chem. 4, 44–53 (2014). https://doi.org/10.4236/gsc.2014.41008
Hagiwara, R., Matsumoto, K., Hwang, J., et al.: Sodium ion batteries using ionic liquids as electrolytes. Chem. Rec. 19, 758–770 (2019). https://doi.org/10.1002/tcr.201800119
Basile, A., Hilder, M., Makhlooghiazad, F., et al.: Sodium energy storage: ionic liquids and organic ionic plastic crystals: advanced electrolytes for safer high performance sodium energy storage technologies. Adv. Energy Mater. 8(17), 1870078 (2018). https://doi.org/10.1002/aenm.201870078
Xu, C.X., Yang, G., Wu, D.X., et al.: Roadmap on ionic liquid electrolytes for energy storage devices. Chem. Asian J. 16, 549–562 (2021). https://doi.org/10.1002/asia.202001414
Wang, Y.M., Song, S.F., Xu, C.H., et al.: Development of solid-state electrolytes for sodium-ion battery: a short review. Nano Mater. Sci. 1, 91–100 (2019). https://doi.org/10.1016/j.nanoms.2019.02.007
Lian, P.J., Zhao, B.S., Zhang, L.Q., et al.: Inorganic sulfide solid electrolytes for all-solid-state lithium secondary batteries. J. Mater. Chem. A 7, 20540–20557 (2019). https://doi.org/10.1039/c9ta04555d
Hu, C.J., Qi, J.Z., Zhang, Y.X., et al.: Room-temperature all-solid-state sodium battery based on bulk interfacial superionic conductor. Nano Lett. 21, 10354–10360 (2021). https://doi.org/10.1021/acs.nanolett.1c03605
Chen, S.L., Che, H.Y., Feng, F., et al.: Poly(vinylene carbonate)-based composite polymer electrolyte with enhanced interfacial stability to realize high-performance room-temperature solid-state sodium batteries. ACS Appl. Mater. Interfaces 11, 43056–43065 (2019). https://doi.org/10.1021/acsami.9b11259
Chen, S.L., Feng, F., Che, H.Y., et al.: High performance solid-state sodium batteries enabled by boron contained 3D composite polymer electrolyte. Chem. Eng. J. 406, 126736 (2021). https://doi.org/10.1016/j.cej.2020.126736
Yao, X.Y., Huang, B.X., Yin, J.Y., et al.: All-solid-state lithium batteries with inorganic solid electrolytes: review of fundamental science. Chin. Phys. B 25, 018802 (2016). https://doi.org/10.1088/1674-1056/25/1/018802
Schnell, J., Günther, T., Knoche, T., et al.: All-solid-state lithium-ion and lithium metal batteries: paving the way to large-scale production. J. Power Sources 382, 160–175 (2018). https://doi.org/10.1016/j.jpowsour.2018.02.062
Xu, G.L., Amine, R., Abouimrane, A., et al.: Challenges in developing electrodes, electrolytes, and diagnostics tools to understand and advance sodium-ion batteries. Adv. Energy Mater. 8, 1702403 (2018). https://doi.org/10.1002/aenm.201702403
Zheng, S.Y., Yan, J.Y., Wang, K.: Engineering research progress of electrochemical microreaction technology: a novel method for electrosynthesis of organic chemicals. Engineering 7, 22–32 (2021). https://doi.org/10.1016/j.eng.2020.06.025
Duchêne, L., Kühnel, R.S., Rentsch, D., et al.: A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 53, 4195–4198 (2017). https://doi.org/10.1039/c7cc00794a
Yang, Z., Jin, M.Y., Cheng, S., et al.: Developing a high-voltage electrolyte based on conjuncto-hydroborates for solid-state sodium batteries. J. Mater. Chem. A 10, 7186–7194 (2022). https://doi.org/10.1039/d1ta09386j
Chen, S.L., Feng, F., Yin, Y.M., et al.: Plastic crystal polymer electrolytes containing boron based anion acceptors for room temperature all-solid-state sodium-ion batteries. Energy Storage Mater. 22, 57–65 (2019). https://doi.org/10.1016/j.ensm.2018.12.023
Zhang, Z.Z., Shao, Y.J., Lotsch, B., et al.: New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 11, 1945–1976 (2018). https://doi.org/10.1039/c8ee01053f
Banerjee, A., Park, K.H., Heo, J.W., et al.: Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. Int. Ed. 55, 9634–9638 (2016). https://doi.org/10.1002/anie.201604158
Kim, J.J., Yoon, K., Park, I., et al.: Progress in the development of sodium-ion solid electrolytes. Small Methods 1, 1700219 (2017). https://doi.org/10.1002/smtd.201700219
Famprikis, T., Canepa, P., Dawson, J.A., et al.: Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18, 1278–1291 (2019). https://doi.org/10.1038/s41563-019-0431-3
Bachman, J.C., Muy, S., Grimaud, A., et al.: Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016). https://doi.org/10.1021/acs.chemrev.5b00563
Lacivita, V., Wang, Y., Bo, S.H., et al.: Ab initio investigation of the stability of electrolyte/electrode interfaces in all-solid-state Na batteries. J. Mater. Chem. A 7, 8144–8155 (2019). https://doi.org/10.1039/c8ta10498k
Lu, X.C., Xia, G.G., Lemmon, J.P., et al.: Advanced materials for sodium-beta alumina batteries: status, challenges and perspectives. J. Power Sources 195, 2431–2442 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.120
Goodenough, J.B.: Evolution of strategies for modern rechargeable batteries. Acc. Chem. Res. 46, 1053–1061 (2013). https://doi.org/10.1021/ar2002705
Lu, Y., Li, L., Zhang, Q., et al.: Electrolyte and interface engineering for solid-state sodium batteries. Joule 2, 1747–1770 (2018). https://doi.org/10.1016/j.joule.2018.07.028
Birnie, D.P., III.: On the structural integrity of the spinel block in the β"-alumina structure. Acta Crystallogr. Sect. B Struct. Sci. 68, 118–122 (2012). https://doi.org/10.1107/s0108768112002649
Kummer, J.T.: Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 29, 2453–2475 (1967). https://doi.org/10.1016/0022-1902(67)80301-4
Sudworth, J.L.: The sodium/sulphur battery. J. Power Sources 11, 143–154 (1984). https://doi.org/10.1016/0378-7753(84)80080-4
Ghadbeigi, L., Szendrei, A., Moreno, P., et al.: Synthesis of iron-doped Na-β″–alumina + yttria-stabilized zirconia composite electrolytes by a vapor phase process. Solid State Ion. 290, 77–82 (2016). https://doi.org/10.1016/j.ssi.2016.04.006
Viswanathan, L., Ikuma, Y., Virkar, A.V.: Transfomation toughening of β″-alumina by incorporation of zirconia. J. Mater. Sci. 18, 109–113 (1983). https://doi.org/10.1007/BF00543815
Liu, Z.H., Chen, J.J., Wang, X.X., et al.: Synthesis and characterization of high ionic-conductive sodium beta-alumina solid electrolyte derived from boehmite. J. Mater. Sci. Mater. Electron. 31, 17670–17678 (2020). https://doi.org/10.1007/s10854-020-04321-7
Li, H., Fan, H.Q., Chen, G.Y., et al.: Performance of nano-3YSZ toughened β’’-alumina solid electrolyte prepared by EDTA-Zr(IV)/Y(III) complex as surface modifier. J. Alloys Compd. 817, 152717 (2020). https://doi.org/10.1016/j.jallcom.2019.152717
Park, R.J.Y., Eschler, C.M., Fincher, C.D., et al.: Semi-solid alkali metal electrodes enabling high critical current densities in solid electrolyte batteries. Nat. Energy 6, 314–322 (2021). https://doi.org/10.1038/s41560-021-00786-w
Spencer Jolly, D., Ning, Z.Y., Darnbrough, J.E., et al.: Sodium/Na β″ alumina interface: effect of pressure on voids. ACS Appl. Mater. Interfaces 12, 678–685 (2020). https://doi.org/10.1021/acsami.9b17786
Lei, D.N., He, Y.B., Huang, H.J., et al.: Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 10, 4244 (2019). https://doi.org/10.1038/s41467-019-11960-w
Medenbach, L., Hartmann, P., Janek, J., et al.: A sodium polysulfide battery with liquid/solid electrolyte: improving sulfur utilization using P2S5 as additive and tetramethylurea as catholyte solvent. Energy Technol. 8, 1901200 (2020). https://doi.org/10.1002/ente.201901200
Wang, D., Hwang, J., Chen, C.Y., et al.: A β″-alumina/inorganic ionic liquid dual electrolyte for intermediate-temperature sodium–sulfur batteries. Adv. Funct. Mater. 31, 2105524 (2021). https://doi.org/10.1002/adfm.202105524
Goodenough, J.B., Hong, H.Y.P., Kafalas, J.A.: Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976). https://doi.org/10.1016/0025-5408(76)90077-5
Hong, H.Y.P.: Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 11, 173–182 (1976). https://doi.org/10.1016/0025-5408(76)90073-8
Zhang, Z.Z., Zou, Z.Y., Kaup, K., et al.: Correlated migration invokes higher Na+-ion conductivity in NaSICON-type solid electrolytes. Adv. Energy Mater. 9, 1902373 (2019). https://doi.org/10.1002/aenm.201902373
Benabed, Y., Rioux, M., Rousselot, S., et al.: Assessing the electrochemical stability window of NASICON-type solid electrolytes. Front. Energy Res. 9, 682008 (2021). https://doi.org/10.3389/fenrg.2021.682008
Schwietert, T.K., Arszelewska, V.A., Wang, C., et al.: Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020). https://doi.org/10.1038/s41563-019-0576-0
Yang, Z.D., Tang, B., Xie, Z.J., et al.: NASICON-type Na3Zr2Si2PO12 solid-state electrolytes for sodium batteries. ChemElectroChem 8, 1035–1047 (2021). https://doi.org/10.1002/celc.202001527
Sun, F., Xiang, Y.X., Sun, Q., et al.: Origin of high ionic conductivity of Sc-doped sodium-rich NASICON solid-state electrolytes. Adv. Funct. Mater. 31, 2102129 (2021). https://doi.org/10.1002/adfm.202102129
Zhang, Z.Z., Zhang, Q.H., Shi, J.N., et al.: A self-forming composite electrolyte for solid-state sodium battery with ultralong cycle life. Adv. Energy Mater. 7, 1601196 (2017). https://doi.org/10.1002/aenm.201601196
Martínez-Cisneros, C.S., Pandit, B., Antonelli, C., et al.: Development of sodium hybrid quasi-solid electrolytes based on porous NASICON and ionic liquids. J. Eur. Ceram. Soc. 41, 7723–7733 (2021). https://doi.org/10.1016/j.jeurceramsoc.2021.08.001
Park, K.H., Bai, Q., Kim, D.H., et al.: Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 8, 1800035 (2018). https://doi.org/10.1002/aenm.201800035
Jansen, M., Henseler, U.: Synthesis, structure determination, and ionic conductivity of sodium tetrathiophosphate. J. Solid State Chem. 99, 110–119 (1992). https://doi.org/10.1016/0022-4596(92)90295-7
Hayashi, A., Noi, K., Sakuda, A., et al.: Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 3, 856 (2012). https://doi.org/10.1038/ncomms1843
Moon, C.K., Lee, H.J., Park, K.H., et al.: Vacancy-driven Na+ superionic conduction in new Ca-doped Na3PS4 for all-solid-state Na-ion batteries. ACS Energy Lett. 3, 2504–2512 (2018). https://doi.org/10.1021/acsenergylett.8b01479
Feng, X.Y., Chien, P.H., Zhu, Z.Y., et al.: Studies of functional defects for fast Na-ion conduction in Na3–yPS4–xClx with a combined experimental and computational approach. Adv. Funct. Mater. 29, 1807951 (2019). https://doi.org/10.1002/adfm.201807951
Han, F.D., Zhu, Y.Z., He, X.F., et al.: Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1501590 (2016). https://doi.org/10.1002/aenm.201501590
Wang, H., Chen, Y., Hood, Z.D., et al.: An air-stable Na3SbS4 superionic conductor prepared by a rapid and economic synthetic procedure. Angew. Chem. Int. Ed. 55, 8551–8555 (2016). https://doi.org/10.1002/anie.201601546
Gamo, H., Phuc, N.H.H., Matsuda, R., et al.: Multiphase Na3SbS4 with high ionic conductivity. Mater. Today Energy 13, 45–49 (2019). https://doi.org/10.1016/j.mtener.2019.04.012
Yubuchi, S., Ito, A., Masuzawa, N., et al.: Aqueous solution synthesis of Na3SbS4–Na2WS4 superionic conductors. J. Mater. Chem. A 8, 1947–1954 (2020). https://doi.org/10.1039/c9ta02246e
Tsuji, F., Masuzawa, N., Sakuda, A., et al.: Preparation and characterization of cation-substituted Na3SbS4 solid electrolytes. ACS Appl. Energy Mater. 3, 11706–11712 (2020). https://doi.org/10.1021/acsaem.0c01823
Banerjee, A., Park, K.H., Heo, J.W., et al.: Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. 128, 9786–9790 (2016). https://doi.org/10.1002/ange.201604158
Tian, Y.S., Sun, Y.Z., Hannah, D.C., et al.: Reactivity-guided interface design in Na metal solid-state batteries. Joule 3, 1037–1050 (2019). https://doi.org/10.1016/j.joule.2018.12.019
Matsuo, M., Kuromoto, S., Sato, T., et al.: Sodium ionic conduction in complex hydrides with [BH4]– and [NH2]– anions. Appl. Phys. Lett. 100, 203904 (2012). https://doi.org/10.1063/1.4716021
Tang, W.S., Yoshida, K., Soloninin, A.V., et al.: Stabilizing superionic-conducting structures via mixed-anion solid solutions of monocarba-closo-borate salts. ACS Energy Lett. 1, 659–664 (2016). https://doi.org/10.1021/acsenergylett.6b00310
Sun, Y.L., Wang, Y.C., Liang, X.M., et al.: Rotational cluster anion enabling superionic conductivity in sodium-rich antiperovskite Na3OBH4. J. Am. Chem. Soc. 141, 5640–5644 (2019). https://doi.org/10.1021/jacs.9b01746
Duchêne, L., Remhof, A., Hagemann, H., et al.: Status and prospects of hydroborate electrolytes for all-solid-state batteries. Energy Storage Mater. 25, 782–794 (2020). https://doi.org/10.1016/j.ensm.2019.08.032
Yoon, K., Kim, J.J., Seong, W.M., et al.: Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in all-solid-state lithium battery. Sci. Rep. 8, 8066 (2018). https://doi.org/10.1038/s41598-018-26101-4
Agrawal, R.C., Pandey, G.P.: Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J. Phys. D Appl. Phys. 41, 223001 (2008). https://doi.org/10.1088/0022-3727/41/22/223001
Long, L.Z., Wang, S.J., Xiao, M., et al.: Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 4, 10038–10069 (2016). https://doi.org/10.1039/c6ta02621d
Wright, P.V.: Electrical conductivity in ionic complexes of poly(ethylene oxide). Brit. Polym. J. 7, 319–327 (1975). https://doi.org/10.1002/pi.4980070505
Xue, Z.G., He, D., Xie, X.L.: Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 3, 19218–19253 (2015). https://doi.org/10.1039/c5ta03471j
Devi, C., Gellanki, J., Pettersson, H., et al.: High sodium ionic conductivity in PEO/PVP solid polymer electrolytes with InAs nanowire fillers. Sci. Rep. 11, 20180 (2021). https://doi.org/10.1038/s41598-021-99663-5
Guo, B., Fu, Y.D., Wang, J.N., et al.: Strategies and characterization methods for achieving high performance PEO-based solid-state lithium-ion batteries. Chem. Commun. 58, 8182–8193 (2022). https://doi.org/10.1039/d2cc02306g
Shenbagavalli, S., Muthuvinayagam, M., Jayanthi, S., et al.: Investigations on Al2O3 dispersed PEO/PVP based Na+ ion conducting blend polymer electrolytes. J. Mater. Sci. Mater. Electron. 32, 9998–10007 (2021). https://doi.org/10.1007/s10854-021-05658-3
Yao, Y.W., Liu, Z.H., Wang, X.X., et al.: Promoted ion conductivity of sodium salt–poly(ethylene oxide) polymer electrolyte induced by adding conductive beta-alumina and application in all-solid-state sodium batteries. J. Mater. Sci. 56, 9951–9960 (2021). https://doi.org/10.1007/s10853-021-05885-3
Chen, G.H., Bai, Y., Gao, Y.S., et al.: Inhibition of crystallization of poly(ethylene oxide) by ionic liquid: insight into plasticizing mechanism and application for solid-state sodium ion batteries. ACS Appl. Mater. Interfaces 11, 43252–43260 (2019). https://doi.org/10.1021/acsami.9b16294
Hulvat, J.F., Stupp, S.I.: Liquid-crystal templating of conducting polymers. Angew. Chem. Int. Ed. 42, 778–781 (2003). https://doi.org/10.1002/anie.200390206
Koduru, H.K., Marinov, Y.G., Hadjichristov, G.B., et al.: Characterization of polymer/liquid crystal composite based electrolyte membranes for sodium ion battery applications. Solid State Ion. 335, 86–96 (2019). https://doi.org/10.1016/j.ssi.2019.02.021
Park, S.S., Tulchinsky, Y., Dincă, M.: Single-ion Li+, Na+, and Mg2+ solid electrolytes supported by a mesoporous anionic Cu–azolate metal–organic framework. J. Am. Chem. Soc. 139, 13260–13263 (2017). https://doi.org/10.1021/jacs.7b06197
Wei, T., Wang, Z.M., Zhang, Q., et al.: Metal–organic framework-based solid-state electrolytes for all solid-state lithium metal batteries: a review. CrystEngComm 24, 5014–5030 (2022). https://doi.org/10.1039/d2ce00663d
Ge, Z., Li, J., Liu, J.: Enhanced electrochemical performance of all-solid-state sodium-sulfur batteries by PEO–NaCF3SO3–MIL-53(Al) solid electrolyte. Ionics 26, 1787–1795 (2020). https://doi.org/10.1007/s11581-020-03513-9
Svarfvar, B.L., Ekman, K.B., Sundell, M.J., et al.: Electron-beam graft-modified membranes with externally controlled flux. Polym. Adv. Technol. 7, 839–846 (1996). https://doi.org/10.1002/(sici)1099-1581(199611)7:11839:aid-pat592%3e3.0.co;2-t
Bristi, A.A., Samson, A.J., Sivakumaran, A., et al.: Ionic conductivity, Na plating–stripping, and battery performance of solid polymer Na ion electrolyte based on poly(vinylidene fluoride) and poly(vinyl pyrrolidone). ACS Appl. Energy Mater. 5, 8812–8822 (2022). https://doi.org/10.1021/acsaem.2c01296
Bag, S., Zhou, C.T., Reid, S., et al.: Electrochemical studies on symmetric solid-state Na-ion full cell using Na3V2(PO4)3 electrodes and polymer composite electrolyte. J. Power Sources 454, 227954 (2020). https://doi.org/10.1016/j.jpowsour.2020.227954
Wang, X.E., Zhu, H.J., Greene, G.W., et al.: Enhancement of ion dynamics in organic ionic plastic crystal/PVDF composite electrolytes prepared by co-electrospinning. J. Mater. Chem. A 4, 9873–9880 (2016). https://doi.org/10.1039/c6ta02817a
Makhlooghiazad, F., Nti, F., Sun, J., et al.: Composite electrolytes based on electrospun PVDF and ionic plastic crystal matrices for Na-metal battery applications. J. Phys. Mater. 4, 034003 (2021). https://doi.org/10.1088/2515-7639/abeed2
Fang, R.Y., Li, Y.T., Wu, N., et al.: Ultra-thin single-particle-layer sodium beta-alumina-based composite polymer electrolyte membrane for sodium-metal batteries. Adv. Funct. Mater. 33, 2211229 (2023). https://doi.org/10.1002/adfm.202211229
Shetty, S.K., Ismayil, Nasreen, et al.: Sodium ion conducting PVA/NaCMC bio poly-blend electrolyte films for energy storage device applications. Int. J. Polym. Anal. Charact. 26, 411–424 (2021). https://doi.org/10.1080/1023666x.2021.1899685
Cyriac, V., Ismayil, Noor, I.S.B.M., et al.: Modification in the microstructure of sodium carboxymethylcellulose/polyvinyl alcohol polyblend films through the incorporation of NaNO3 for energy storage applications. Int. J. Energy Res. 46, 22845–22866 (2022). https://doi.org/10.1002/er.8588
Yu, X.W., Xue, L.G., Goodenough, J.B., et al.: All-solid-state sodium batteries with a polyethylene glycol diacrylate–Na3Zr2Si2PO12 composite electrolyte. Adv. Energy Sustain. Res. 2, 2000061 (2021). https://doi.org/10.1002/aesr.202000061
Ren, Y.X., Hortance, N., McBride, J., et al.: Sodium-sulfur batteries enabled by a protected inorganic/organic hybrid solid electrolyte. ACS Energy Lett. 6, 345–353 (2021). https://doi.org/10.1021/acsenergylett.0c02494
Batten, S.R., Champness, N.R., Chen, X.M., et al.: Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 85, 1715–1724 (2013). https://doi.org/10.1351/pac-rec-12-11-20
Gebert, F., Knott, J., Gorkin, R., et al.: Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 36, 10–30 (2021). https://doi.org/10.1016/j.ensm.2020.11.030
Menisha, M., Senavirathna, S.L.N., Vignarooban, K., et al.: Synthesis, electrochemical and optical studies of poly(ethylene oxide) based gel-polymer electrolytes for sodium-ion secondary batteries. Solid State Ion. 371, 115755 (2021). https://doi.org/10.1016/j.ssi.2021.115755
Feuillade, G., Perche, P.: Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 5, 63–69 (1975). https://doi.org/10.1007/BF00625960
Yu, Q.P., Lu, Q.W., Qi, X.G., et al.: Liquid electrolyte immobilized in compact polymer matrix for stable sodium metal anodes. Energy Storage Mater. 23, 610–616 (2019). https://doi.org/10.1016/j.ensm.2019.03.011
Choi, N.S., Lee, Y.G., Park, J.K., et al.: Preparation and electrochemcial characteristics of plasticized polymer electrolytes based upon a P(VdF-co-HFP)/PVAc blend. Electrochim. Acta 46, 1581–1586 (2001). https://doi.org/10.1016/S0013-4686(00)00756-8
Vo, D.T., Do, H.N., Nguyen, T.T., et al.: Sodium ion conducting gel polymer electrolyte using poly(vinylidene fluoride hexafluoropropylene). Mater. Sci. Eng. B 241, 27–35 (2019). https://doi.org/10.1016/j.mseb.2019.02.007
Janakiraman, S., Agrawal, A., Biswal, R., et al.: An amorphous polyvinylidene fluoride-co-hexafluoropropylene based gel polymer electrolyte for sodium-ion cells. Appl. Surf. Sci. Adv. 6, 100139 (2021). https://doi.org/10.1016/j.apsadv.2021.100139
Chauhan, A.K., Kumar, D., Mishra, K., et al.: Performance enhancement of Na+ ion conducting porous gel polymer electrolyte using NaAlO2 active filler. Mater. Today Commun. 26, 101713 (2021). https://doi.org/10.1016/j.mtcomm.2020.101713
Kwon, D.S., Gong, S.H., Yun, S., et al.: Regulating Na electrodeposition by sodiophilic grafting onto porosity-gradient gel polymer electrolytes for dendrite-free sodium metal batteries. ACS Appl. Mater. Interfaces 14, 47650–47658 (2022). https://doi.org/10.1021/acsami.2c12287
Zhao, C.D., Guo, J.Z., Gu, Z.Y., et al.: Flexible quasi-solid-state sodium-ion full battery with ultralong cycle life, high energy density and high-rate capability. Nano Res. 15, 925–932 (2022). https://doi.org/10.1007/s12274-021-3577-7
Shubha, N., Prasanth, R., Hng, H.H., et al.: Study on effect of poly (ethylene oxide) addition and in-situ porosity generation on poly (vinylidene fluoride)-glass ceramic composite membranes for lithium polymer batteries. J. Power Sources 267, 48–57 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.074
Zhang, Y.G., Bakenov, Z., Tan, T.Z., et al.: Polyacrylonitrile-nanofiber-based gel polymer electrolyte for novel aqueous sodium-ion battery based on a Na4Mn9O18 cathode and Zn metal anode. Polymers 10, 853 (2018). https://doi.org/10.3390/polym10080853
Lonchakova, O.V., Semenikhin, O.A., Zakharkin, M.V., et al.: Efficient gel-polymer electrolyte for sodium-ion batteries based on poly(acrylonitrile-co-methyl acrylate). Electrochim. Acta 334, 135512 (2020). https://doi.org/10.1016/j.electacta.2019.135512
Zhou, Y.N., Xiao, Z.C., Han, D.Z., et al.: Approaching practically accessible and environmentally adaptive sodium metal batteries with high loading cathodes through in situ interlock interface. Adv. Funct. Mater. 32, 2111314 (2022). https://doi.org/10.1002/adfm.202111314
Shuai, Y., Lou, J., Pei, X.L., et al.: Constructing an in situ polymer electrolyte and a Na-rich artificial SEI layer toward practical solid-state Na metal batteries. ACS Appl. Mater. Interfaces 14, 45382–45391 (2022). https://doi.org/10.1021/acsami.2c12518
Gandini, A.: Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 41, 9491–9504 (2008). https://doi.org/10.1021/ma801735u
Mittal, N., Ojanguren, A., Cavasin, N., et al.: Transient rechargeable battery with a high lithium transport number cellulosic separator. Adv. Funct. Mater. 31, 2101827 (2021). https://doi.org/10.1002/adfm.202101827
Mittal, N., Tien, S.A., Lizundia, E., et al.: Hierarchical nanocellulose-based gel polymer electrolytes for stable Na electrodeposition in sodium ion batteries. Small 18, 2107183 (2022). https://doi.org/10.1002/smll.202107183
Yang, Z.G., Zhang, J.L., Kintner-Meyer, M.C.W., et al.: Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011). https://doi.org/10.1021/cr100290v
Simari, C., Tuccillo, M., Brutti, S., et al.: Sodiated Nafion membranes for sodium metal aprotic batteries. Electrochim. Acta 410, 139936 (2022). https://doi.org/10.1016/j.electacta.2022.139936
Abouimrane, A., Whitfield, P.S., Niketic, S., et al.: Investigation of Li salt doped succinonitrile as potential solid electrolytes for lithium batteries. J. Power Sources 174, 883–888 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.103
Kim, S.H., Choi, K.H., Cho, S.J., et al.: A shape-deformable and thermally stable solid-state electrolyte based on a plastic crystal composite polymer electrolyte for flexible/safer lithium-ion batteries. J. Mater. Chem. A 2, 10854–10861 (2014). https://doi.org/10.1039/c4ta00494a
Abu-Lebdeh, Y., Abouimrane, A., Alarco, P.J., et al.: Ambient temperature proton conducting plastic crystal electrolytes. Electrochem. Commun. 6, 432–434 (2004). https://doi.org/10.1016/j.elecom.2004.02.015
Long, S.: Fast ion conduction in molecular plastic crystals. Solid State Ion. 161, 105–112 (2003). https://doi.org/10.1016/s0167-2738(03)00208-x
Zhu, X.M., Zhao, R.R., Deng, W.W., et al.: An all-solid-state and all-organic sodium-ion battery based on redox-active polymers and plastic crystal electrolyte. Electrochim. Acta 178, 55–59 (2015). https://doi.org/10.1016/j.electacta.2015.07.163
Yu, X.W., Xue, L.G., Goodenough, J.B., et al.: Ambient-temperature all-solid-state sodium batteries with a laminated composite electrolyte. Adv. Funct. Mater. 31, 2002144 (2021). https://doi.org/10.1002/adfm.202002144
Makhlooghiazad, F., Gunzelmann, D., Hilder, M., et al.: Mixed phase solid-state plastic crystal electrolytes based on a phosphonium cation for sodium devices. Adv. Energy Mater. 7, 1601272 (2017). https://doi.org/10.1002/aenm.201601272
Biernacka, K., Makhlooghiazad, F., Popov, I., et al.: Investigation of unusual conductivity behavior and ion dynamics in hexamethylguanidinium bis(fluorosulfonyl)imide-based electrolytes for sodium batteries. J. Phys. Chem. C 125, 12518–12530 (2021). https://doi.org/10.1021/acs.jpcc.1c01777
Makhlooghiazad, F., Sharma, M., Zhang, Z.Z., et al.: Stable high-temperature cycling of Na metal batteries on Na3V2(PO4)3 and Na2FeP2O7 cathodes in NaFSI-rich organic ionic plastic crystal electrolytes. J. Phys. Chem. Lett. 11, 2092–2100 (2020). https://doi.org/10.1021/acs.jpclett.0c00149
Acknowledgements
This work was supported by the Natural Science Foundation of China (22005190, 21938005), the Science & Technology Commission of Shanghai Municipality (20QB1405700, 19DZ1205500), and the Zhejiang Key Research and Development Program (2020C01128).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, S., Che, H., Chen, S. et al. Research Progress on the Solid Electrolyte of Solid-State Sodium-Ion Batteries. Electrochem. Energy Rev. 7, 3 (2024). https://doi.org/10.1007/s41918-023-00196-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-023-00196-4