Abstract
Electrocatalytic water splitting driven by renewable energy input to produce clean hydrogen (H2) has been widely considered a prospective approach for a future hydrogen-based society. However, the development of industrial alkaline water electrolyzers is hindered due to their unfavorable thermodynamics with high overpotential for delivering the whole process, caused by sluggish kinetics involving four-electron transfer. Further exploration of water electrolysis with low energy consumption and high efficiency is urgently required to meet the ever-growing energy storage and portfolio demands. This review emphasizes the strategies proposed thus far to pursue high-efficiency water electrolysis systems, including from the aspects of electrocatalysts (from monofunctional to bifunctional), electrode engineering (from powdery to self-supported), energy sources (from nonrenewable to renewable), electrolytes (from pure to hybrid), and cell configurations (from integrated to decoupled). Critical appraisals of the pivotal electrochemistry are highlighted to address the challenges in elevating the overall efficiency of water splitting. Finally, valuable insights for the future development directions and bottlenecks of advanced, sustainable, and high-efficiency water splitting systems are outlined.
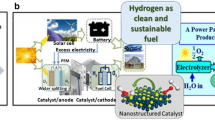
Graphical abstract



Reproduced with permissions from Ref. [4]. Copyright © 2020, Springer. Reproduced with permissions from Ref. [24]. Copyright © 1959, the American Chemical Society. Reproduced with permissions from Ref. [25]. Copyright © 2009, Elsevier. Reproduced with permissions from Ref. [26]. Copyright © 2017, Elsevier. Reproduced with permissions from Ref. [27]. Copyright © 2018, Elsevier. Reproduced with permissions from Ref. [28]. Copyright © 2018, Elsevier. Reproduced with permissions from Ref. [29]. Copyright © 2008, Elsevier. Reproduced with permissions from Ref. [30]. Copyright © 2020, the American Chemical Society. Reproduced with permissions from Ref. [31]. Copyright © 2020, the Royal Society of Chemistry. Reproduced with permissions from Ref. [32]. Copyright © 2021, John Wiley and Sons












Similar content being viewed by others
References
Chu, S., Majumdar, A.: Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012). https://doi.org/10.1038/nature11475
Turner, J.A.: Sustainable hydrogen production. Science 305, 972–974 (2004). https://doi.org/10.1126/science.1103197
You, B., Sun, Y.J.: Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 51, 1571–1580 (2018). https://doi.org/10.1021/acs.accounts.8b00002
Li, X., Zhao, L.L., Yu, J.Y., et al.: Water splitting: from electrode to green energy system. Nano Micro Lett. 12, 1–29 (2020). https://doi.org/10.1007/s40820-020-00469-3
Sun, H., Yan, Z., Liu, F., et al.: Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 32, e1806326 (2020). https://doi.org/10.1002/adma.201806326
Hu, Q., Li, G.M., Han, Z., et al.: Recent progress in the hybrids of transition metals/carbon for electrochemical water splitting. J. Mater. Chem. A 7, 14380–14390 (2019). https://doi.org/10.1039/c9ta04163j
You, X., Bin, Z.: Recent advances in electrochemical hydrogen production from water assisted by alternative oxidation reactions. ChemElectroChem 6, 3214–3226 (2019). https://doi.org/10.1002/celc.201900675
Wu, D., Wei, Y.C., Ren, X., et al.: Co(OH)2 nanoparticle-encapsulating conductive nanowires array: room-temperature electrochemical preparation for high-performance water oxidation electrocatalysis. Adv. Mater. 30, 1705366 (2018). https://doi.org/10.1002/adma.201705366
Weng, C.C., Ren, J.T., Yuan, Z.Y.: Transition metal phosphide-based materials for efficient electrochemical hydrogen evolution: a critical review. Chemsuschem 13, 3357–3375 (2020). https://doi.org/10.1002/cssc.202000416
Ren, J.T., Yao, Y.L., Yuan, Z.Y.: Fabrication strategies of porous precious-metal-free bifunctional electrocatalysts for overall water splitting: recent advances. Green Energy Environ. 6, 620–643 (2021). https://doi.org/10.1016/j.gee.2020.11.023
Lv, X.W., Weng, C.C., Zhu, Y.P., et al.: Nanoporous metal phosphonate hybrid materials as a novel platform for emerging applications: a critical review. Small 17, e2005304 (2021). https://doi.org/10.1002/smll.202005304
Ma, T.Y., Dai, S., Jaroniec, M., et al.: Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 136, 13925–13931 (2014). https://doi.org/10.1021/ja5082553
Wang, J., Cui, W., Liu, Q., et al.: Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 28, 215–230 (2016). https://doi.org/10.1002/adma.201502696
Lv, X.W., Hu, Z.P., Ren, J.T., et al.: Self-supported Al-doped cobalt phosphide nanosheets grown on three-dimensional Ni foam for highly efficient water reduction and oxidation. Inorg. Chem. Front. 6, 74–81 (2019). https://doi.org/10.1039/c8qi01026a
Yang, Y., Lun, Z.Y., Xia, G.L., et al.: Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 8, 3563–3571 (2015). https://doi.org/10.1039/c5ee02460a
Lv, X.W., Ren, J.T., Wang, Y.S., et al.: Well-defined phase-controlled cobalt phosphide nanoparticles encapsulated in nitrogen-doped graphitized carbon shell with enhanced electrocatalytic activity for hydrogen evolution reaction at all-pH. ACS Sustain. Chem. Eng. 7, 8993–9001 (2019). https://doi.org/10.1021/acssuschemeng.9b01263
Ren, J.T., Chen, L., Weng, C.C., et al.: Well-defined Mo2C nanoparticles embedded in porous N-doped carbon matrix for highly efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 10, 33276–33286 (2018). https://doi.org/10.1021/acsami.8b12108
Song, Y.J., Yuan, Z.Y.: One-pot synthesis of Mo2N/NC catalysts with enhanced electrocatalytic activity for hydrogen evolution reaction. Electrochim. Acta 246, 536–543 (2017). https://doi.org/10.1016/j.electacta.2017.06.086
Xu, S.R., Zhao, H.T., Li, T.S., et al.: Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: recent advances and future prospects. J. Mater. Chem. A 8, 19729–19745 (2020). https://doi.org/10.1039/d0ta05628f
McAllister, J., Bandeira, N.A.G., McGlynn, J.C., et al.: Tuning and mechanistic insights of metal chalcogenide molecular catalysts for the hydrogen-evolution reaction. Nat. Commun. 10, 370 (2019). https://doi.org/10.1038/s41467-018-08208-4
Hu, C.L., Zhang, L., Gong, J.L.: Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 12, 2620–2645 (2019). https://doi.org/10.1039/c9ee01202h
Shinagawa, T., Takanabe, K.: Towards versatile and sustainable hydrogen production through electrocatalytic water splitting: electrolyte engineering. Chemsuschem 10, 1318–1336 (2017). https://doi.org/10.1002/cssc.201601583
Kwon, J., Han, H., Choi, S., et al.: Current status of self-supported catalysts for robust and efficient water splitting for commercial electrolyzer. ChemCatChem 11, 5898–5912 (2019). https://doi.org/10.1002/cctc.201901638
Rashid, M.M., Mesfer, M.K.A., Naseem, H., et al.: Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 4, 2249–8958 (2015)
Marangio, F., Santarelli, M., Calì, M.: Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrog. Energy 34, 1143–1158 (2009). https://doi.org/10.1016/j.ijhydene.2008.11.083
Feng, Q., Yuan, X.Z., Liu, G.Y., et al.: A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 366, 33–55 (2017). https://doi.org/10.1016/j.jpowsour.2017.09.006
Vincent, I., Choi, B., Nakoji, M., et al.: Pulsed current water splitting electrochemical cycle for hydrogen production. Int. J. Hydrog. Energy 43, 10240–10248 (2018). https://doi.org/10.1016/j.ijhydene.2018.04.087
Ju, H., Badwal, S., Giddey, S.: A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 231, 502–533 (2018). https://doi.org/10.1016/j.apenergy.2018.09.125
Ni, M., Leung, M.K.H., Leung, D.Y.C.: Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrog. Energy 33, 2337–2354 (2008). https://doi.org/10.1016/j.ijhydene.2008.02.048
Zhu, J., Hu, L.S., Zhao, P.X., et al.: Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 120, 851–918 (2020). https://doi.org/10.1021/acs.chemrev.9b00248
Wang, J., Gao, Y., Kong, H., et al.: Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 49, 9154–9196 (2020). https://doi.org/10.1039/d0cs00575d
Yu, Z.Y., Duan, Y., Feng, X.Y., et al.: Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Adv. Mater. 33, e2007100 (2021). https://doi.org/10.1002/adma.202007100
Xie, L., Zhang, R., Cui, L., et al.: High-performance electrolytic oxygen evolution in neutral media catalyzed by a cobalt phosphate nanoarray. Angew. Chem. Int. Ed. 56, 1064–1068 (2017). https://doi.org/10.1002/anie.201610776
Zhao, H., Yuan, Z.Y.: Insights into transition metal phosphate materials for efficient electrocatalysis. ChemCatChem 12, 3797–3810 (2020). https://doi.org/10.1002/cctc.202000360
Su, J., Ge, R., Jiang, K., et al.: Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: highly robust electrocatalysts for oxygen evolution in acidic media. Adv. Mater. (2018). https://doi.org/10.1002/adma.201801351
Song, F., Bai, L., Moysiadou, A., et al.: Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748–7759 (2018). https://doi.org/10.1021/jacs.8b04546
Jahan, M., Liu, Z.L., Loh, K.P.: A graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 23, 5363–5372 (2013). https://doi.org/10.1002/adfm.201300510
Zhao, G.Q., Rui, K., Dou, S.X., et al.: Heterostructures for electrochemical hydrogen evolution reaction: a review. Adv. Funct. Mater. 28, 1803291 (2018). https://doi.org/10.1002/adfm.201803291
Strmcnik, D., Lopes, P.P., Genorio, B., et al.: Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 29, 29–36 (2016). https://doi.org/10.1016/j.nanoen.2016.04.017
Callejas, J.F., Read, C.G., Roske, C.W., et al.: Synthesis, characterization, and properties of metal phosphide catalysts for the hydrogen-evolution reaction. Chem. Mater. 28, 6017–6044 (2016). https://doi.org/10.1021/acs.chemmater.6b02148
Zhang, H., Ma, Z., Duan, J., et al.: Active sites implanted carbon cages in core-shell architecture: highly active and durable electrocatalyst for hydrogen evolution reaction. ACS Nano 10, 684–694 (2016). https://doi.org/10.1021/acsnano.5b05728
Peng, Y., Chen, S.W.: Electrocatalysts based on metal@carbon core@shell nanocomposites: an overview. Green Energy Environ. 3, 335–351 (2018). https://doi.org/10.1016/j.gee.2018.07.006
Lv, X.W., Tian, W.W., Liu, Y.P., et al.: Well-defined CoP/Ni2P nanohybrids encapsulated in a nitrogen-doped carbon matrix as advanced multifunctional electrocatalysts for efficient overall water splitting and zinc–air batteries. Mater. Chem. Front. 3, 2428–2436 (2019). https://doi.org/10.1039/c9qm00449a
Ma, Y.Y., Wu, C.X., Feng, X.J., et al.: Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 10, 788–798 (2017). https://doi.org/10.1039/c6ee03768b
Zhang, X.L., Hu, S.J., Zheng, Y.R., et al.: Polymorphic cobalt diselenide as extremely stable electrocatalyst in acidic media via a phase-mixing strategy. Nat. Commun. 10, 5338 (2019). https://doi.org/10.1038/s41467-019-12992-y
Li, S.Z., Zang, W.J., Liu, X.M., et al.: Heterojunction engineering of MoSe2/MoS2 with electronic modulation towards synergetic hydrogen evolution reaction and supercapacitance performance. Chem. Eng. J. 359, 1419–1426 (2019). https://doi.org/10.1016/j.cej.2018.11.036
Yang, Z.X., He, R., Wu, H.M., et al.: Needle-like CoP/rGO growth on nickel foam as an efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 46, 9690–9698 (2021). https://doi.org/10.1016/j.ijhydene.2020.07.114
Wang, J.Y., Ting, O.Y., Li, N., et al.: S, N co-doped carbon nanotube-encapsulated core-shelled CoS2@Co nanoparticles: efficient and stable bifunctional catalysts for overall water splitting. Sci. Bull. 63, 1130–1140 (2018). https://doi.org/10.1016/j.scib.2018.07.008
Liu, Q., Tian, J.Q., Cui, W., et al.: Carbon nanotubes decorated with CoP nanocrystals: a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. Engl. 53, 6710–6714 (2014). https://doi.org/10.1002/anie.201404161
Lv, X.W., Hu, Z.P., Chen, L., et al.: Organic–inorganic metal phosphonate-derived nitrogen-doped core-shell Ni2P nanoparticles supported on Ni foam for efficient hydrogen evolution reaction at all pH values. ACS Sustain. Chem. Eng. 7, 12770–12778 (2019). https://doi.org/10.1021/acssuschemeng.9b01355
Han, C., Li, W.J., Shu, C.Z., et al.: Catalytic activity boosting of nickel sulfide toward oxygen evolution reaction via confined overdoping engineering. ACS Appl. Energy Mater. 2, 5363–5372 (2019). https://doi.org/10.1021/acsaem.9b00932
Zhu, K., Zhu, X., Yang, W.: Application of in situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts. Angew. Chem. Int. Ed. Engl. 58, 1252–1265 (2019). https://doi.org/10.1002/anie.201802923
Mohammed-Ibrahim, J., Sun, X.M.: Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting - a review. J. Energy Chem. 34, 111–160 (2019). https://doi.org/10.1016/j.jechem.2018.09.016
Gong, L.Q., Yang, H., Douka, A.I., et al.: Recent progress on NiFe-based electrocatalysts for alkaline oxygen evolution. Adv. Sustain. Syst. 5, 2000136 (2021). https://doi.org/10.1002/adsu.202000136
Danilovic, N., Subbaraman, R., Chang, K.C., et al.: Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 5, 2474–2478 (2014). https://doi.org/10.1021/jz501061n
McCrory, C.C.L., Jung, S., Ferrer, I.M., et al.: Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015). https://doi.org/10.1021/ja510442p
Lim, J., Park, D., Jeon, S.S., et al.: Ultrathin IrO2 nanoneedles for electrochemical water oxidation. Adv. Funct. Mater. 28, 1704796 (2018). https://doi.org/10.1002/adfm.201704796
Shi, Q.R., Zhu, C.Z., Du, D., et al.: Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 48, 3181–3192 (2019). https://doi.org/10.1039/c8cs00671g
Danilovic, N., Subbaraman, R., Chang, K.C., et al.: Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew. Chem. Int. Ed. Engl. 53, 14016–14021 (2014). https://doi.org/10.1002/anie.201406455
Jamesh, M.I.: Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Sources 333, 213–236 (2016). https://doi.org/10.1016/j.jpowsour.2016.09.161
Zeng, F., Broicher, C., Hofmann, J.P., et al.: Facile synthesis of sulfur-containing transition metal (Mn, Fe, Co, and Ni) (hydr)oxides for efficient oxygen evolution reaction. ChemCatChem 12, 710–716 (2020). https://doi.org/10.1002/cctc.201901493
Si, C.H., Zhang, Y.L., Zhang, C.Q., et al.: Mesoporous nanostructured spinel-type MFe2O4 (M = Co, Mn, Ni) oxides as efficient bi-functional electrocatalysts towards oxygen reduction and oxygen evolution. Electrochim. Acta 245, 829–838 (2017). https://doi.org/10.1016/j.electacta.2017.06.029
Ashok, A., Kumar, A., Bhosale, R.R., et al.: Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 809, 22–30 (2018). https://doi.org/10.1016/j.jelechem.2017.12.043
Ren, J.T., Yuan, G.G., Weng, C.C., et al.: Uniquely integrated Fe-doped Ni(OH)2 nanosheets for highly efficient oxygen and hydrogen evolution reactions. Nanoscale 10, 10620–10628 (2018). https://doi.org/10.1039/c8nr01655k
Ren, J.T., Yuan, G.G., Weng, C.C., et al.: Rationally designed Co3O4-C nanowire arrays on Ni foam derived from metal organic framework as reversible oxygen evolution electrodes with enhanced performance for Zn-air batteries. ACS Sustain. Chem. Eng. 6, 707–718 (2018). https://doi.org/10.1021/acssuschemeng.7b03034
Lu, X.Y., Zhao, C.: Highly efficient and robust oxygen evolution catalysts achieved by anchoring nanocrystalline cobalt oxides onto mildly oxidized multiwalled carbon nanotubes. J. Mater. Chem. A 1, 12053 (2013). https://doi.org/10.1039/c3ta12912h
Li, S.L., Li, Z.C., Ma, R.G., et al.: A glass-ceramic with accelerated surface reconstruction toward the efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 60, 3773–3780 (2021). https://doi.org/10.1002/anie.202014210
Han, X.P., He, G.W., He, Y., et al.: Engineering catalytic active sites on cobalt oxide surface for enhanced oxygen electrocatalysis. Adv. Energy Mater. 8, 1870043 (2018). https://doi.org/10.1002/aenm.201870043
Xu, Q., Jiang, H., Duan, X., et al.: Fluorination-enabled reconstruction of NiFe electrocatalysts for efficient water oxidation. Nano Lett. 21, 492–499 (2021). https://doi.org/10.1021/acs.nanolett.0c03950
Wang, Y., Zhu, Y.L., Zhao, S.L., et al.: Anion etching for accessing rapid and deep self-reconstruction of precatalysts for water oxidation. Matter 3, 2124–2137 (2020). https://doi.org/10.1016/j.matt.2020.09.016
Liu, X., Meng, J.S., Ni, K., et al.: Complete reconstruction of hydrate pre-catalysts for ultrastable water electrolysis in industrial-concentration alkali media. Cell Rep. Phys. Sci. 1, 100241 (2020). https://doi.org/10.1016/j.xcrp.2020.100241
Fan, K., Zou, H., Lu, Y., et al.: Direct observation of structural evolution of metal chalcogenide in electrocatalytic water oxidation. ACS Nano 12, 12369–12379 (2018). https://doi.org/10.1021/acsnano.8b06312
Cheng, F.P., Li, Z.J., Wang, L., et al.: In situ identification of the electrocatalytic water oxidation behavior of a nickel-based metal-organic framework nanoarray. Mater. Horiz. 8, 556–564 (2021). https://doi.org/10.1039/d0mh01757d
Shi, Y.M., Du, W., Zhou, W., et al.: Unveiling the promotion of surface-adsorbed chalcogenate on the electrocatalytic oxygen evolution reaction. Angew. Chem. Int. Ed. 132, 22656–22660 (2020). https://doi.org/10.1002/ange.202011097
Zhu, Y.P., Liu, Y.P., Ren, T.Z., et al.: Self-supported cobalt phosphide mesoporous nanorod arrays: a flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv. Funct. Mater. 25, 7337–7347 (2015). https://doi.org/10.1002/adfm.201503666
Cobo, S., Heidkamp, J., Jacques, P.A., et al.: A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 11, 802–807 (2012). https://doi.org/10.1038/nmat3385
Gerken, J.B., McAlpin, J.G., Chen, J.Y.C., et al.: Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 133, 14431–14442 (2011). https://doi.org/10.1021/ja205647m
Tang, C., Cheng, N., Pu, Z., et al.: NiSe nanowire film supported on nickel foam: an efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. Int. Ed. Engl. 54, 9351–9355 (2015). https://doi.org/10.1002/anie.201503407
Jin, H., Joo, J., Chaudhari, N.K., et al.: Recent progress in bifunctional electrocatalysts for overall water splitting under acidic conditions. ChemElectroChem 6, 3244–3253 (2019). https://doi.org/10.1002/celc.201900507
Yu, J.M., Le, T.A., Tran, N.Q., et al.: Earth-abundant transition-metal-based bifunctional electrocatalysts for overall water splitting in alkaline media. Chem. Eur. J. 26, 6423–6436 (2020). https://doi.org/10.1002/chem.202000209
Sun, Y.Q., Zhang, T., Li, C.C., et al.: Compositional engineering of sulfides, phosphides, carbides, nitrides, oxides, and hydroxides for water splitting. J. Mater. Chem. A 8, 13415–13436 (2020). https://doi.org/10.1039/d0ta05038e
Xiong, B.Y., Chen, L.S., Shi, J.L.: Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 8, 3688–3707 (2018). https://doi.org/10.1021/acscatal.7b04286
Zhao, H., Zhu, Y.P., Yuan, Z.Y.: Three-dimensional electrocatalysts for sustainable water splitting reactions. Eur. J. Inorg. Chem. 2016, 1916–1923 (2016). https://doi.org/10.1002/ejic.201501181
Wang, Z.C., Liu, H.L., Ge, R.X., et al.: Phosphorus-doped Co3O4 nanowire array: a highly efficient bifunctional electrocatalyst for overall water splitting. ACS Catal. 8, 2236–2241 (2018). https://doi.org/10.1021/acscatal.7b03594
Zhang, H.J., Maijenburg, A.W., Li, X.P., et al.: Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting. Adv. Funct. Mater. 30, 2003261 (2020). https://doi.org/10.1002/adfm.202003261
Li, H., Wen, P., Itanze, D.S., et al.: Phosphorus-rich colloidal cobalt diphosphide (CoP2) nanocrystals for electrochemical and photoelectrochemical hydrogen evolution. Adv. Mater. 31, e1900813 (2019). https://doi.org/10.1002/adma.201900813
Xu, H.B., Jia, H.X., Fei, B., et al.: Charge transfer engineering via multiple heteroatom doping in dual carbon-coupled cobalt phosphides for highly efficient overall water splitting. Appl. Catal. B Environ. 268, 118404 (2020). https://doi.org/10.1016/j.apcatb.2019.118404
Lv, X.W., Weng, C.C., Yuan, Z.Y.: Ambient ammonia electrosynthesis: current status, challenges, and perspectives. Chemsuschem 13, 3061–3078 (2020). https://doi.org/10.1002/cssc.202000670
Yue, X., Huang, S.L., Cai, J.J., et al.: Heteroatoms dual doped porous graphene nanosheets as efficient bifunctional metal-free electrocatalysts for overall water-splitting. J. Mater. Chem. A 5, 7784–7790 (2017). https://doi.org/10.1039/C7TA01957B
Lv, X.W., Liu, Y.P., Tian, W.W., et al.: Aluminum and phosphorus codoped “superaerophobic” Co3O4 microspheres for highly efficient electrochemical water splitting and Zn-air batteries. J. Energy Chem. 50, 324–331 (2020). https://doi.org/10.1016/j.jechem.2020.02.055
Yang, W.Q., Zeng, J.R., Hua, Y.X., et al.: Defect engineering of cobalt microspheres by S doping and electrochemical oxidation as efficient bifunctional and durable electrocatalysts for water splitting at high current densities. J. Power Sources 436, 226887 (2019). https://doi.org/10.1016/j.jpowsour.2019.226887
Zhao, Y.X., Chang, C., Teng, F., et al.: Defect-engineered ultrathin δ-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting. Adv. Energy Mater. 7, 1700005 (2017). https://doi.org/10.1002/aenm.201770102
Wu, T., Dong, C.L., Sun, D., et al.: Enhancing electrocatalytic water splitting by surface defect engineering in two-dimensional electrocatalysts. Nanoscale 13, 1581–1595 (2021). https://doi.org/10.1039/d0nr08009h
Han, N., Liu, P.Y., Jiang, J., et al.: Recent advances in nanostructured metal nitrides for water splitting. J. Mater. Chem. A 6, 19912–19933 (2018). https://doi.org/10.1039/c8ta06529b
Yan, H., Tian, C., Wang, L., et al.: Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. Engl. 54, 6325–6329 (2015). https://doi.org/10.1002/anie.201501419
Zhang, Y.Q., Ouyang, B., Xu, J., et al.: Rapid synthesis of cobalt nitride nanowires: highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. 128, 8812–8816 (2016). https://doi.org/10.1002/ange.201604372
Jacob, K., Verma, R., Mallya, R.: Nitride synthesis using ammonia and hydrazine: a thermodynamic panorama. J. Mater. Sci. 37, 4465–4472 (2002)
Ma, L., Ting, L.R.L., Molinari, V., et al.: Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A 3, 8361–8368 (2015). https://doi.org/10.1039/c5ta00139k
Ren, J.T., Song, Y.J., Yuan, Z.Y.: Facile synthesis of molybdenum carbide nanoparticles in situ decorated on nitrogen-doped porous carbons for hydrogen evolution reaction. J. Energy Chem. 32, 78–84 (2019). https://doi.org/10.1016/j.jechem.2018.07.006
Ai, L.H., Su, J.F., Wang, M., et al.: Bamboo-structured nitrogen-doped carbon nanotube coencapsulating cobalt and molybdenum carbide nanoparticles: an efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 6, 9912–9920 (2018). https://doi.org/10.1021/acssuschemeng.8b01120
Yu, Y.D., Zhou, J., Sun, Z.M.: Novel 2D transition-metal carbides: ultrahigh performance electrocatalysts for overall water splitting and oxygen reduction. Adv. Funct. Mater. 30, 2070311 (2020). https://doi.org/10.1002/adfm.202070311
Zhang, H., Yang, X.H., Zhang, H.J., et al.: Transition-metal carbides as hydrogen evolution reduction electrocatalysts: synthetic methods and optimization strategies. Chem. Eur. J. 27, 5074–5090 (2021). https://doi.org/10.1002/chem.202003979
Qiao, L.L., Zhu, A.Q., Zeng, W.X., et al.: Achieving electronic structure reconfiguration in metallic carbides for robust electrochemical water splitting. J. Mater. Chem. A 8, 2453–2462 (2020). https://doi.org/10.1039/c9ta10682k
Guo, Y., Park, T., Yi, J.W., et al.: Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 31, e1807134 (2019). https://doi.org/10.1002/adma.201807134
Gao, Q., Zhang, W., Shi, Z., et al.: Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv. Mater. 31, e1802880 (2019). https://doi.org/10.1002/adma.201802880
Jia, L.N., Li, C., Zhao, Y.R., et al.: Interfacial engineering of Mo2C-Mo3C2 heteronanowires for high performance hydrogen evolution reactions. Nanoscale 11, 23318–23329 (2019). https://doi.org/10.1039/c9nr08986a
Han, N., Yang, K.R., Lu, Z., et al.: Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 9, 924 (2018). https://doi.org/10.1038/s41467-018-03429-z
Houston, J.E., Laramore, G.E., Park, R.L.: Surface electronic properties of tungsten, tungsten carbide, and platinum. Science 185, 258–260 (1974). https://doi.org/10.1126/science.185.4147.258
Huang, J.B., Jiang, Y., An, T.Y., et al.: Increasing the active sites and intrinsic activity of transition metal chalcogenide electrocatalysts for enhanced water splitting. J. Mater. Chem. A 8, 25465–25498 (2020). https://doi.org/10.1039/d0ta08802a
Wei, S.T., Cui, X.Q., Xu, Y.C., et al.: Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 4, 368–374 (2019). https://doi.org/10.1021/acsenergylett.8b01840
Wang, M., Zhang, L., He, Y.J., et al.: Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 9, 5320–5363 (2021). https://doi.org/10.1039/d0ta12152e
Ren, J.T., Yuan, Z.Y.: Hierarchical nickel sulfide nanosheets directly grown on Ni foam: a stable and efficient electrocatalyst for water reduction and oxidation in alkaline medium. ACS Sustain. Chem. Eng. 5, 7203–7210 (2017). https://doi.org/10.1021/acssuschemeng.7b01419
Xia, X.Y., Wang, L.J., Sui, N., et al.: Recent progress in transition metal selenide electrocatalysts for water splitting. Nanoscale 12, 12249–12262 (2020). https://doi.org/10.1039/d0nr02939d
Peng, X., Yan, Y.J., Jin, X., et al.: Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 78, 105234 (2020). https://doi.org/10.1016/j.nanoen.2020.105234
Qi, H.Y., Zhang, P., Wang, H.Y., et al.: Cu2Se nanowires shelled with NiFe layered double hydroxide nanosheets for overall water-splitting. J. Colloid Interface Sci. 599, 370–380 (2021). https://doi.org/10.1016/j.jcis.2021.04.101
Wan, S., Jin, W.Y., Guo, X.L., et al.: Self-templating construction of porous CoSe2 nanosheet arrays as efficient bifunctional electrocatalysts for overall water splitting. ACS Sustain. Chem. Eng. 6, 15374–15382 (2018). https://doi.org/10.1021/acssuschemeng.8b03804
He, X.P., Gao, B., Wang, G.B., et al.: A new nanocomposite: carbon cloth based polyaniline for an electrochemical supercapacitor. Electrochim. Acta 111, 210–215 (2013). https://doi.org/10.1016/j.electacta.2013.07.226
Pandey, G.P., Rastogi, A.C., Westgate, C.R.: All-solid-state supercapacitors with poly(3,4-ethylenedioxythiophene)-coated carbon fiber paper electrodes and ionic liquid gel polymer electrolyte. J. Power Sources 245, 857–865 (2014). https://doi.org/10.1016/j.jpowsour.2013.07.017
Wei, S., Wang, X.X., Wang, J.M., et al.: CoS2 nanoneedle array on Ti mesh: a stable and efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Electrochim. Acta 246, 776–782 (2017). https://doi.org/10.1016/j.electacta.2017.06.068
Li, C.P., Shi, X.D., Liang, S.Q., et al.: Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem. Eng. J. 379, 122248 (2020). https://doi.org/10.1016/j.cej.2019.122248
Zhang, R., Tang, C., Kong, R., et al.: Al-doped CoP nanoarray: a durable water-splitting electrocatalyst with superhigh activity. Nanoscale 9, 4793–4800 (2017). https://doi.org/10.1039/c7nr00740j
Du, H., Xia, L., Zhu, S., et al.: Al-doped Ni2P nanosheet array: a superior and durable electrocatalyst for alkaline hydrogen evolution. Chem. Commun. Camb. Engl. 54, 2894–2897 (2018). https://doi.org/10.1039/c7cc09445k
Tang, C., Gan, L., Zhang, R., et al.: Ternary FexCo1−xP nanowire array as a robust hydrogen evolution reaction electrocatalyst with Pt-like activity: experimental and theoretical insight. Nano Lett. 16, 6617–6621 (2016). https://doi.org/10.1021/acs.nanolett.6b03332
Hu, Z.H., Zhang, L., Huang, J.T., et al.: Self-supported nickel-doped molybdenum carbide nanoflower clusters on carbon fiber paper for an efficient hydrogen evolution reaction. Nanoscale 13, 8264–8274 (2021). https://doi.org/10.1039/d1nr00169h
Barati Darband, G., Lotfi, N., Aliabadi, A., et al.: Hydrazine-assisted electrochemical hydrogen production by efficient and self-supported electrodeposited Ni–Cu–P@Ni–Cu nano-micro dendrite catalyst. Electrochim. Acta 382, 138335 (2021). https://doi.org/10.1016/j.electacta.2021.138335
Wang, X.G., Li, W., Xiong, D.H., et al.: Bifunctional catalysts: bifunctional nickel phosphide nanocatalysts supported on carbon fiber paper for highly efficient and stable overall water splitting. Adv. Funct. Mater. 26, 4066–4077 (2016). https://doi.org/10.1002/adfm.201670146
Li, Y.J., Zhang, H.C., Jiang, M., et al.: 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting. Adv. Funct. Mater. 27, 1702513 (2017). https://doi.org/10.1002/adfm.201702513
Jin, H.Y., Wang, J., Su, D.F., et al.: In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 137, 2688–2694 (2015). https://doi.org/10.1021/ja5127165
Tian, J.Q., Cheng, N.Y., Liu, Q., et al.: Self-supported NiMo hollow nanorod array: an efficient 3D bifunctional catalytic electrode for overall water splitting. J. Mater. Chem. A 3, 20056–20059 (2015). https://doi.org/10.1039/c5ta04723d
Yuan, H.F., Wang, S.M., Gu, X.D., et al.: One-step solid-phase boronation to fabricate self-supported porous FeNiB/FeNi foam for efficient electrocatalytic oxygen evolution and overall water splitting. J. Mater. Chem. A 7, 19554–19564 (2019). https://doi.org/10.1039/c9ta04076e
Feng, L.L., Yu, G.T., Wu, Y.Y., et al.: High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 137, 14023–14026 (2015). https://doi.org/10.1021/jacs.5b08186
Zhang, J., Wang, T., Pohl, D., et al.: Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 128, 6814–6819 (2016). https://doi.org/10.1002/ange.201602237
Czioska, S., Wang, J.Y., Teng, X., et al.: Hierarchically structured CuCo2S4 nanowire arrays as efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 6, 11877–11883 (2018). https://doi.org/10.1021/acssuschemeng.8b02155
Xue, Z.L., Kang, J.Y., Guo, D., et al.: Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 273, 229–238 (2018). https://doi.org/10.1016/j.electacta.2018.04.056
Yan, F., Wang, Y., Li, K., et al.: Highly stable three-dimensional porous nickel-iron nitride nanosheets for full water splitting at high current densities. Chemistry 23, 10187–10194 (2017). https://doi.org/10.1002/chem.201701662
Lu, Y.K., Li, Z.X., Xu, Y.L., et al.: Bimetallic Co-Mo nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 411, 128433 (2021). https://doi.org/10.1016/j.cej.2021.128433
Yu, L., Zhou, H.Q., Sun, J.Y., et al.: Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 10, 1820–1827 (2017). https://doi.org/10.1039/c7ee01571b
Liu, B., Wang, Y., Peng, H.Q., et al.: Iron vacancies induced bifunctionality in ultrathin feroxyhyte nanosheets for overall water splitting. Adv. Mater. (2018). https://doi.org/10.1002/adma.201803144
Jiang, P., Liu, Q., Liang, Y., et al.: A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew. Chem. Int. Ed. 53, 12855–12859 (2014). https://doi.org/10.1002/anie.201406848
Tian, J., Liu, Q., Cheng, N., et al.: Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. Int. Ed. Engl. 53, 9577–9581 (2014). https://doi.org/10.1002/anie.201403842
Zhang, J., Wang, T., Liu, P., et al.: Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 8, 15437 (2017). https://doi.org/10.1038/ncomms15437
Sun, H.M., Tian, C.Y., Li, Y.L., et al.: Coupling NiCo alloy and CeO2 to enhance electrocatalytic hydrogen evolution in alkaline solution. Adv. Sustain. Syst. 4, 2000122 (2020). https://doi.org/10.1002/adsu.202000122
Gao, M.Y., Yang, C., Zhang, Q.B., et al.: Facile electrochemical preparation of self-supported porous Ni–Mo alloy microsphere films as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 5, 5797–5805 (2017). https://doi.org/10.1039/c6ta10812a
Ni, L., Zhou, J.H., Chen, N.N., et al.: In situ direct growth of flower-like hierarchical architecture of CoNi-layered double hydroxide on Ni foam as an efficient self-supported oxygen evolution electrocatalyst. Int. J. Hydrog. Energy 45, 22788–22796 (2020). https://doi.org/10.1016/j.ijhydene.2020.06.139
Zhong, H.H., Cheng, X.K., Xu, H.T., et al.: Carbon fiber paper supported interlayer space enlarged Ni2Fe-LDHs improved OER electrocatalytic activity. Electrochim. Acta 258, 554–560 (2017). https://doi.org/10.1016/j.electacta.2017.11.098
Xu, J., Wang, M.S., Yang, F., et al.: Self-supported porous Ni–Fe–W hydroxide nanosheets on carbon fiber: a highly efficient electrode for oxygen evolution reaction. Inorg. Chem. 58, 13037–13048 (2019). https://doi.org/10.1021/acs.inorgchem.9b01953
Zhou, Q.Q., Wang, J.Y., Guo, F.Y., et al.: Self-supported bimetallic phosphide-carbon nanostructures derived from metal-organic frameworks as bifunctional catalysts for highly efficient water splitting. Electrochim. Acta 318, 244–251 (2019). https://doi.org/10.1016/j.electacta.2019.06.082
Jiang, D.L., Ma, W.X., Yang, R., et al.: Nickel–manganese bimetallic phosphides porous nanosheet arrays as highly active bifunctional hydrogen and oxygen evolution electrocatalysts for overall water splitting. Electrochim. Acta 329, 135121 (2020). https://doi.org/10.1016/j.electacta.2019.135121
Xuan, C.J., Peng, Z.K., Xia, K.D., et al.: Self-supported ternary Ni–Fe–P nanosheets derived from metal-organic frameworks as efficient overall water splitting electrocatalysts. Electrochim. Acta 258, 423–432 (2017). https://doi.org/10.1016/j.electacta.2017.11.078
Rajesh, J.A., Jo, I.R., Kang, S.H., et al.: Potentiostatically deposited bimetallic cobalt-nickel selenide nanostructures on nickel foam for highly efficient overall water splitting. Int. J. Hydrog. Energy 46, 7297–7308 (2021). https://doi.org/10.1016/j.ijhydene.2020.11.252
Yang, H.H., Huang, Y., Teoh, W.Y., et al.: Molybdenum selenide nanosheets surrounding nickel selenides sub-microislands on nickel foam as high-performance bifunctional electrocatalysts for water splitting. Electrochim. Acta 349, 136336 (2020). https://doi.org/10.1016/j.electacta.2020.136336
Zhang, F.F., Pei, Y., Ge, Y.C., et al.: Controlled synthesis of eutectic NiSe/Ni3Se2 self-supported on Ni foam: an excellent bifunctional electrocatalyst for overall water splitting. Adv. Mater. Interfaces 5, 1701507 (2018). https://doi.org/10.1002/admi.201701507
Ren, J.T., Hu, Z.P., Chen, C., et al.: Integrated Ni2P nanosheet arrays on three-dimensional Ni foam for highly efficient water reduction and oxidation. J. Energy Chem. 26, 1196–1202 (2017). https://doi.org/10.1016/j.jechem.2017.07.016
Li, C.Q., Zhao, X., Liu, Y.W., et al.: 3D Ni–Co sulfoxide nanosheet arrays electrodeposited on Ni foam: a bifunctional electrocatalyst towards efficient and stable water splitting. Electrochim. Acta 292, 347–356 (2018). https://doi.org/10.1016/j.electacta.2018.06.159
Meng, T., Hao, Y.N., Zheng, L.R., et al.: Organophosphoric acid-derived CoP quantum dots@S, N-codoped graphite carbon as a trifunctional electrocatalyst for overall water splitting and Zn-air batteries. Nanoscale 10, 14613–14626 (2018). https://doi.org/10.1039/c8nr03299h
Yan, L., Xu, Y., Chen, P., et al.: A freestanding 3D heterostructure film stitched by MOF-derived carbon nanotube microsphere superstructure and reduced graphene oxide sheets: a superior multifunctional electrode for overall water splitting and Zn-air batteries. Adv. Mater. 32, e2003313 (2020). https://doi.org/10.1002/adma.202003313
Tee, S.Y., Win, K.Y., Teo, W.S., et al.: Recent progress in energy-driven water splitting. Adv. Sci. 4, 1600337 (2017). https://doi.org/10.1002/advs.201600337
Long, X.F., Gao, L.L., Li, F., et al.: Bamboo shoots shaped FeVO4 passivated ZnO nanorods photoanode for improved charge separation/transfer process towards efficient solar water splitting. Appl. Catal. B Environ. 257, 117813 (2019). https://doi.org/10.1016/j.apcatb.2019.117813
He, T., Zu, L., Zhang, Y., et al.: Amorphous semiconductor nanowires created by site-specific heteroatom substitution with significantly enhanced photoelectrochemical performance. ACS Nano 10, 7882–7891 (2016). https://doi.org/10.1021/acsnano.6b03801
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). https://doi.org/10.1038/238037a0
Stoerzinger, K.A., Wang, L., Ye, Y.F., et al.: Linking surface chemistry to photovoltage in Sr-substituted LaFeO3 for water oxidation. J. Mater. Chem. A 6, 22170–22178 (2018). https://doi.org/10.1039/c8ta05741a
Gurudayal, A., Sabba, D., Kumar, M.H., et al.: Perovskite-hematite tandem cells for efficient overall solar driven water splitting. Nano Lett. 15, 3833–3839 (2015). https://doi.org/10.1021/acs.nanolett.5b00616
Swierk, J.R., Mallouk, T.E.: Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells. Chem. Soc. Rev. 42, 2357–2387 (2013). https://doi.org/10.1039/c2cs35246j
Shi, X.J., Zhang, K., Shin, K., et al.: Unassisted photoelectrochemical water splitting beyond 5.7% solar-to-hydrogen conversion efficiency by a wireless monolithic photoanode/dye-sensitised solar cell tandem device. Nano Energy 13, 182–191 (2015). https://doi.org/10.1016/j.nanoen.2015.02.018
Kim, D.W., Kim, J.U., Shin, S.S., et al.: Facile one-pot synthesis of self-assembled quantum-rod TiO2 spheres with enhanced charge transport properties for dye-sensitized solar cells and solar water-splitting. J. Alloys Compd. 697, 222–230 (2017). https://doi.org/10.1016/j.jallcom.2016.12.112
Xu, C., Wang, X.D., Wang, Z.L.: Nanowire structured hybrid cell for concurrently scavenging solar and mechanical energies. J. Am. Chem. Soc. 131, 5866–5872 (2009). https://doi.org/10.1021/ja810158x
Yang, Z., Deng, J., Sun, H., et al.: Self-powered energy fiber: energy conversion in the sheath and storage in the core. Adv. Mater. 26, 7038–7042 (2014). https://doi.org/10.1002/adma.201401972
Abdi, F.F., Han, L., Smets, A.H.M., et al.: Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 4, 2195 (2013). https://doi.org/10.1038/ncomms3195
Meng, X.G., Wang, T., Liu, L.Q., et al.: Photothermal conversion of CO2 into CH4 with H2 over Group VIII nanocatalysts: an alternative approach for solar fuel production. Angew. Chem. Int. Ed. 53, 11478–11482 (2014). https://doi.org/10.1002/anie.201404953
Zhang, X.F., Gao, W.Q., Su, X.W., et al.: Conversion of solar power to chemical energy based on carbon nanoparticle modified photo-thermoelectric generator and electrochemical water splitting system. Nano Energy 48, 481–488 (2018). https://doi.org/10.1016/j.nanoen.2018.03.055
Zhao, L.L., Yang, Z.Y., Cao, Q., et al.: An earth-abundant and multifunctional Ni nanosheets array as electrocatalysts and heat absorption layer integrated thermoelectric device for overall water splitting. Nano Energy 56, 563–570 (2019). https://doi.org/10.1016/j.nanoen.2018.11.035
Yuan, H.F., Liu, F., Xue, G.B., et al.: Laser patterned and bifunctional Ni@N-doped carbon nanotubes as electrocatalyst and photothermal conversion layer for water splitting driven by thermoelectric device. Appl. Catal. B Environ. 283, 119647 (2021). https://doi.org/10.1016/j.apcatb.2020.119647
Huang, X., Zhang, W., Guan, G., et al.: Design and functionalization of the NIR-responsive photothermal semiconductor nanomaterials for cancer theranostics. Acc. Chem. Res. 50, 2529–2538 (2017). https://doi.org/10.1021/acs.accounts.7b00294
Yang, W., Ahn, J., Oh, Y., et al.: Adjusting the anisotropy of 1D Sb2Se3 nanostructures for highly efficient photoelectrochemical water splitting. Adv. Energy Mater. 8, 1702888 (2018). https://doi.org/10.1002/aenm.201702888
Getoff, N.: Basic problems of photochemical and photoelectrochemical hydrogen production from water. Int. J. Hydrog. Energy 9, 997–1004 (1984)
Shin, S.M., Jung, J.Y., Park, M.J., et al.: Catalyst-free hydrogen evolution of Si photocathode by thermovoltage-driven solar water splitting. J. Power Sources 279, 151–156 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.020
Meng, F.L., Yilmaz, G., Ding, T.P., et al.: A hybrid solar absorber-electrocatalytic N-doped carbon/alloy/semiconductor electrode for localized photothermic electrocatalysis. Adv. Mater. 31, 1903605 (2019). https://doi.org/10.1002/adma.201903605
Wei, A.M., Xie, X.K., Wen, Z., et al.: Triboelectric nanogenerator driven self-powered photoelectrochemical water splitting based on hematite photoanodes. ACS Nano 12, 8625–8632 (2018). https://doi.org/10.1021/acsnano.8b04363
Ren, X.H., Fan, H.Q., Wang, C., et al.: Wind energy harvester based on coaxial rotatory freestanding triboelectric nanogenerators for self-powered water splitting. Nano Energy 50, 562–570 (2018). https://doi.org/10.1016/j.nanoen.2018.06.002
Kong, D., Zheng, Y., Kobielusz, M., et al.: Recent advances in visible light-driven water oxidation and reduction in suspension systems. Mater. Today 21, 897–924 (2018). https://doi.org/10.1016/j.mattod.2018.04.009
You, B., Liu, X., Jiang, N., et al.: A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 138, 13639–13646 (2016). https://doi.org/10.1021/jacs.6b07127
Cui, X., Chen, M.L., Xiong, R., et al.: Ultrastable and efficient H2 production via membrane-free hybrid water electrolysis over a bifunctional catalyst of hierarchical Mo-Ni alloy nanoparticles. J. Mater. Chem. A 7, 16501–16507 (2019). https://doi.org/10.1039/c9ta03924d
Hu, S.N., Tan, Y., Feng, C.Q., et al.: Synthesis of N doped NiZnCu-layered double hydroxides with reduced graphene oxide on nickel foam as versatile electrocatalysts for hydrogen production in hybrid-water electrolysis. J. Power Sources 453, 227872 (2020). https://doi.org/10.1016/j.jpowsour.2020.227872
Sun, H.Y., Xu, G.R., Li, F.M., et al.: Hydrogen generation from ammonia electrolysis on bifunctional platinum nanocubes electrocatalysts. J. Energy Chem. 47, 234–240 (2020). https://doi.org/10.1016/j.jechem.2020.01.035
Gao, Y., Wang, Q., He, T., et al.: Defective crystalline molybdenum phosphides as bifunctional catalysts for hydrogen evolution and hydrazine oxidation reactions during water splitting. Inorg. Chem. Front. 6, 2686–2695 (2019). https://doi.org/10.1039/c9qi01005j
Qian, Q.Z., Zhang, J.H., Li, J.M., et al.: Artificial heterointerfaces achieve delicate reaction kinetics towards hydrogen evolution and hydrazine oxidation catalysis. Angew. Chem. 133, 6049–6058 (2021). https://doi.org/10.1002/ange.202014362
Sun, Q.Q., Li, Y.B., Wang, J.F., et al.: Pulsed electrodeposition of well-ordered nanoporous Cu-doped Ni arrays promotes high-efficiency overall hydrazine splitting. J. Mater. Chem. A 8, 21084–21093 (2020). https://doi.org/10.1039/d0ta08078k
Liu, X., He, J., Zhao, S., et al.: Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 9, 4365 (2018). https://doi.org/10.1038/s41467-018-06815-9
Zhang, J.Y., Tian, X.N., He, T., et al.: In situ formation of Ni3Se4 nanorod arrays as versatile electrocatalysts for electrochemical oxidation reactions in hybrid water electrolysis. J. Mater. Chem. A 6, 15653–15658 (2018). https://doi.org/10.1039/c8ta06361c
Yu, Z.Y., Lang, C.C., Gao, M.R., et al.: Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 11, 1890–1897 (2018). https://doi.org/10.1039/c8ee00521d
Xu, H.Z., Ye, K., Zhu, K., et al.: Template-directed assembly of urchin-like CoSx/Co-MOF as an efficient bifunctional electrocatalyst for overall water and urea electrolysis. Inorg. Chem. Front. 7, 2602–2610 (2020). https://doi.org/10.1039/d0qi00408a
Sun, H.C., Zhang, W., Li, J.G., et al.: Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B Environ. 284, 119740 (2021). https://doi.org/10.1016/j.apcatb.2020.119740
Yu, L., Wu, L.B., McElhenny, B., et al.: Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 13, 3439–3446 (2020). https://doi.org/10.1039/d0ee00921k
Wang, C.Z., Zhu, M.Z., Cao, Z.Y., et al.: Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B Environ. 291, 120071 (2021). https://doi.org/10.1016/j.apcatb.2021.120071
Zhao, Y.Q., Jin, B., Zheng, Y., et al.: Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 8, 1801926 (2018). https://doi.org/10.1002/aenm.201801926
Lv, Q.L., Han, J.X., Tan, X.L., et al.: Featherlike NiCoP holey nanoarrys for efficient and stable seawater splitting. ACS Appl. Energy Mater. 2, 3910–3917 (2019). https://doi.org/10.1021/acsaem.9b00599
Chen, Y.X., Lavacchi, A., Miller, H.A., et al.: Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis. Nat. Commun. 5, 4036 (2014). https://doi.org/10.1038/ncomms5036
Huang, Y., Chong, X., Liu, C., et al.: Boosting hydrogen production by anodic oxidation of primary amines over a NiSe nanorod electrode. Angew. Chem. Int. Ed. Engl. 57, 13163–13166 (2018). https://doi.org/10.1002/anie.201807717
Qian, Q.Z., Li, Y.P., Liu, Y., et al.: Hierarchical multi-component nanosheet array electrode with abundant NiCo/MoNi4 heterostructure interfaces enables superior bifunctionality towards hydrazine oxidation assisted energy-saving hydrogen generation. Chem. Eng. J. 414, 128818 (2021). https://doi.org/10.1016/j.cej.2021.128818
Wang, Z.Y., Xu, L., Huang, F.Z., et al.: Copper-nickel nitride nanosheets as efficient bifunctional catalysts for hydrazine-assisted electrolytic hydrogen production. Adv. Energy Mater. 9, 1900390 (2019). https://doi.org/10.1002/aenm.201900390
Sun, Q.Q., Wang, L.Y., Shen, Y.Q., et al.: Bifunctional copper-doped nickel catalysts enable energy-efficient hydrogen production via hydrazine oxidation and hydrogen evolution reduction. ACS Sustain. Chem. Eng. 6, 12746–12754 (2018). https://doi.org/10.1021/acssuschemeng.8b01887
Tang, C., Zhang, R., Lu, W., et al.: Energy-saving electrolytic hydrogen generation: Ni2P nanoarray as a high-performance non-noble-metal electrocatalyst. Angew. Chem. Int. Ed. Engl. 56, 842–846 (2017). https://doi.org/10.1002/anie.201608899
Zhang, J., Liu, Y., Li, J., et al.: Vanadium substitution steering reaction kinetics acceleration for Ni3N nanosheets endows exceptionally energy-saving hydrogen evolution coupled with hydrazine oxidation. ACS Appl. Mater. Interfaces 13, 3881–3890 (2021). https://doi.org/10.1021/acsami.0c18684
Li, Y., Li, J., Qian, Q., et al.: Superhydrophilic Ni-based multicomponent nanorod-confined-nanoflake array electrode achieves waste-battery-driven hydrogen evolution and hydrazine oxidation. Small 17, e2008148 (2021). https://doi.org/10.1002/smll.202008148
Li, Y.P., Zhang, J.H., Liu, Y., et al.: Partially exposed RuP2 surface in hybrid structure endows its bifunctionality for hydrazine oxidation and hydrogen evolution catalysis. Sci. Adv. 6, eabb4197 (2020). https://doi.org/10.1126/sciadv.abb4197
Liu, Y., Zhang, J., Li, Y., et al.: Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production. Nat. Commun. 11, 1853 (2020). https://doi.org/10.1038/s41467-020-15563-8
Chen, S., Duan, J.J., Vasileff, A., et al.: Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angew. Chem. 128, 3868–3872 (2016). https://doi.org/10.1002/ange.201600387
Dresp, S., Dionigi, F., Klingenhof, M., et al.: Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019). https://doi.org/10.1021/acsenergylett.9b00220
Wang, H.Y., Weng, C.C., Ren, J.T., et al.: An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 15, 1408–1426 (2021). https://doi.org/10.1007/s11705-021-2102-6
Paul, A., Symes, M.D.: Decoupled electrolysis for water splitting. Curr. Opin. Green Sustain. Chem. 29, 100453 (2021). https://doi.org/10.1016/j.cogsc.2021.100453
McHugh, P.J., Stergiou, A.D., Symes, M.D.: Decoupled electrochemical water splitting: from fundamentals to applications. Adv. Energy Mater. 10, 2002453 (2020). https://doi.org/10.1002/aenm.202002453
Li, W., Jiang, N., Hu, B., et al.: Electrolyzer design for flexible decoupled water splitting and organic upgrading with electron reservoirs. Chem 4, 637–649 (2018). https://doi.org/10.1016/j.chempr.2017.12.019
Huang, J.H., Wang, Y.G.: Efficient renewable-to-hydrogen conversion via decoupled electrochemical water splitting. Cell Rep. Phys. Sci. 1, 100138 (2020). https://doi.org/10.1016/j.xcrp.2020.100138
Dotan, H., Landman, A., Sheehan, S.W., et al.: Decoupled hydrogen and oxygen evolution by a two-step electrochemical–chemical cycle for efficient overall water splitting. Nat. Energy 4, 786–795 (2019). https://doi.org/10.1038/s41560-019-0462-7
Symes, M.D., Cronin, L.: Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 5, 403–409 (2013). https://doi.org/10.1038/nchem.1621
Chen, J.J., Symes, M.D., Cronin, L.: Highly reduced and protonated aqueous solutions of [P2W18O62]6− for on-demand hydrogen generation and energy storage. Nat. Chem. 10, 1042–1047 (2018). https://doi.org/10.1038/s41557-018-0109-5
Rausch, B., Symes, M.D., Chisholm, G., et al.: Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 345, 1326–1330 (2014). https://doi.org/10.1126/science.1257443
Ho, A., Zhou, X.H., Han, L.H., et al.: Decoupling H2(g) and O2(g) production in water splitting by a solar-driven V3+/2+(aq, H2SO4)|KOH(aq) cell. ACS Energy Lett. 4, 968–976 (2019). https://doi.org/10.1021/acsenergylett.9b00278
Zhao, Y., Ding, C.M., Zhu, J., et al.: A hydrogen farm strategy for scalable solar hydrogen production with particulate photocatalysts. Angew. Chem. 132, 9740–9745 (2020). https://doi.org/10.1002/ange.202001438
Rausch, B., Symes, M.D., Cronin, L.: A bio-inspired, small molecule electron-coupled-proton buffer for decoupling the half-reactions of electrolytic water splitting. J. Am. Chem. Soc. 135, 13656–13659 (2013). https://doi.org/10.1021/ja4071893
Nudehi, S., Larson, C., Prusinski, W., et al.: Solar thermal decoupled water electrolysis process II: an extended investigation of the anodic electrochemical reaction. Chem. Eng. Sci. 181, 159–172 (2018). https://doi.org/10.1016/j.ces.2017.12.032
Kirkaldy, N., Chisholm, G., Chen, J.J., et al.: A practical, organic-mediated, hybrid electrolyser that decouples hydrogen production at high current densities. Chem. Sci. 9, 1621–1626 (2018). https://doi.org/10.1039/c7sc05388f
Liu, Z.C., Zhang, G., Zhang, K., et al.: Low electronegativity Mn bulk doping intensifies charge storage of Ni2P redox shuttle for membrane-free water electrolysis. J. Mater. Chem. A 8, 4073–4082 (2020). https://doi.org/10.1039/c9ta10213b
Chen, L., Dong, X., Wang, Y., et al.: Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat. Commun. 7, 11741 (2016). https://doi.org/10.1038/ncomms11741
Guo, M.R., Wang, L., Zhan, J., et al.: A novel design of an electrolyser using a trifunctional (HER/OER/ORR) electrocatalyst for decoupled H2/O2 generation and solar to hydrogen conversion. J. Mater. Chem. A 8, 16609–16615 (2020). https://doi.org/10.1039/d0ta05102k
Jin, Z., Li, P., Xiao, D.: A hydrogen-evolving hybrid-electrolyte battery with electrochemical/photoelectrochemical charging from water oxidation. Chemsuschem 10, 483–488 (2017). https://doi.org/10.1002/cssc.201601317
Wang, J.Y., Ji, L.L., Teng, X., et al.: Decoupling half-reactions of electrolytic water splitting by integrating a polyaniline electrode. J. Mater. Chem. A 7, 13149–13153 (2019). https://doi.org/10.1039/c9ta03285a
Ma, Y., Dong, X., Wang, Y., et al.: Decoupling hydrogen and oxygen production in acidic water electrolysis using a polytriphenylamine-based battery electrode. Angew. Chem. Int. Ed. Engl. 57, 2904–2908 (2018). https://doi.org/10.1002/anie.201800436
Ma, Y., Guo, Z., Dong, X., et al.: Organic proton-buffer electrode to separate hydrogen and oxygen evolution in acid water electrolysis. Angew. Chem. Int. Ed. Engl. 58, 4622–4626 (2019). https://doi.org/10.1002/anie.201814625
Amstutz, V., Toghill, K.E., Powlesland, F., et al.: Renewable hydrogen generation from a dual-circuit redox flow battery. Energy Environ. Sci. 7, 2350–2358 (2014). https://doi.org/10.1039/c4ee00098f
Goodwin, S., Walsh, D.A.: Closed bipolar electrodes for spatial separation of H2 and O2 evolution during water electrolysis and the development of high-voltage fuel cells. ACS Appl. Mater. Interfaces 9, 23654–23661 (2017). https://doi.org/10.1021/acsami.7b04226
Wu, Z.X., Zhao, Y., Jin, W., et al.: Recent progress of vacancy engineering for electrochemical energy conversion related applications. Adv. Funct. Mater. 31, 2009070 (2021). https://doi.org/10.1002/adfm.202009070
Wu, Z.X., Zhao, Y., Wu, H.B., et al.: Corrosion engineering on iron foam toward efficiently electrocatalytic overall water splitting powered by sustainable energy. Adv. Funct. Mater. 31, 2010437 (2021). https://doi.org/10.1002/adfm.202010437
Xu, S.S., Lv, X.W., Zhao, Y.M., et al.: Engineering morphologies of cobalt oxide/phosphate-carbon nanohybrids for high-efficiency electrochemical water oxidation and reduction. J. Energy Chem. 52, 139–146 (2021). https://doi.org/10.1016/j.jechem.2020.04.054
Ma, T.Y., Cao, J.L., Jaroniec, M., et al.: Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew. Chem. 128, 1150–1154 (2016). https://doi.org/10.1002/ange.201509758
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22179065, 21875118, 22111530112), the Tianjin Research Innovation Project for Postgraduate Students (2020YJSB143), and the Ph.D. Candidate Research Innovation Fund of NKU School of Materials Science and Engineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, XW., Tian, WW. & Yuan, ZY. Recent Advances in High-Efficiency Electrocatalytic Water Splitting Systems. Electrochem. Energy Rev. 6, 23 (2023). https://doi.org/10.1007/s41918-022-00159-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00159-1