Abstract
Localized electrochemical deposition microadditive manufacturing (AM) (LECD-µAM) technology represents a nontraditional manufacturing method applied for the layer-by-layer fabrication of metal microstructures via a fully automatic feedback mechanism. In terms of material utilization and complex structure formation, the proposed technology exhibits great potential for microstructure fabrication. The LECD-µAM technology introduced in this study involves the reduction of metal cations in the electrolyte to form metal microstructures. This study showed the flow simulation of an electrolyte in the cantilever probe and pressure distribution at the probe tip. In addition, the effect of extrusion pressure on the deposition structure was investigated experimentally. Combined with the experimental results, we discussed the effects of LECD-µAM technology on deposition outcomes and optimized parameters and designed a printing route for the deposition of complex metal microstructure arrays with smooth surfaces. The proposed technology attained a deposition rate and a microstructural copper content of 0.961 µm/s and 99.5%. In addition, LECD-µAM technology can be performed at room temperature, has low environmental requirements and cost, provides a good deposition surface, and holds great potential for the manufacture of three-dimensional and other complex microstructure arrays.
Highlights
-
1.
The technology realizes micron level complex structure additive manufacturing based on the hollow atomic force microscopy (AFM) cantilever probe.
-
2.
By designing the deposition path, adjust the extrusion pressure to control the deposition structure.
-
3.
The proposed technology attained a deposition rate and a microstructural copper content of 0.961 µm/s and 99.5%. It has the characteristics of faster speed and higher purity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Miniaturized parts with additive manufacturing (AM) of metal microstructure arrays have found wide applications as core carriers in aerospace, microelectronics packaging, microsensors, and biomedicine [1]. Common manufacturing methods for metal microcolumn array structures include precision machining technology, microelectric discharge wire-cutting technology, electrochemical machining technology, LIGA (from the German Lithographie, Galvanoformung, Abformung) technology, and UV LIGA technology. Traditional removal methods, such as microdischarge wire-cutting technology, are relatively complex, exhibit low productivity, and experience difficulty in the formation of complex structures [2]. AM refers to the process of layer-by-layer material addition to creating miniaturized parts with a desired structure. AM technologies, such as electrochemical machining technology, show the advantages of convenient design, low cost, environmental protection, and broad prospects for microstructure fabrication [3].
Common AM methods, such as stereolithography (SLA) [4] and selective laser sintering, are widely used [5,6,7,8,9,10,11,12]. Local fusion of metal particles occurs through the application of a high-energy beam to metal nanopowder, with the target structure formed through layer-by-layer stacking. Although the applications of these techniques are arguably simple and cost-effective, rapid temperature fluctuations during the stacking process cause them to exhibit suboptimal mechanical properties and adversely affect the quality of the metal microstructure. Consequently, considerable deformations, voids, and cracks may manifest on the structure’s surface. Selective laser melting [13, 14] and selective electron beam melting [15] are also utilized in the AM of metals. Despite the achievability of parts with high density and superior performance, the minimum achievable line width falls within the range of tens of microns due to the metal powder’s particle diameter and the metal’s high heat dissipation performance.
The primary methods for AM of microscale three-dimensional (3D) structures comprise micro-SLA, two-photon polymerization laser 3D direct writing, electrospray printing, and electrochemical deposition AM. The electrochemical deposition AM technology is based on the principle of an electrochemical reaction involving the reduction of metal cations in the electrolyte to form a metal microstructure [16, 17]. Compared with other methods, this process shows no residual stress nor strain and presents higher efficiency, lower cost, and more classification options. Electrochemical deposition AM technologies are primarily categorized into two types: masked and nonfilm electrodeposition. The former is mainly based on LIGA and EFAB technologies. LIGA technology utilizes polymethyl methacrylate (PMMA) as a photoresist and high-energy X-rays as an exposure source, which results in the formation of 3D metal microstructures through photolithography, electroformation, and replication. Numerous researchers have successfully used LIGA technology to develop micro-optical devices [18], Ni microarrays for drug delivery [19], and PMMA honeycomb structures with a high aspect ratio [20]. The EFAB technology attains the superposition of metal microstructures through layer-by-layer and cyclic layer deposition. Alper et al. [21] applied EFAB technology to fabricate microgyroscopes with a high aspect ratio and incorporated them with complex microstructures, such as gears and microsurgical tools [22]. However, the mask electrodeposition process is complex and costly, and mask preparation suffers from its own complications. Therefore, scholars should aspire the fabricate microstructures for the formation of 3D complex microstructures.
Without the use of a mask, the electrodeposition process exhibits high formability and relatively low cost and eliminates the need for a mask or support structure. Such a condition enables localized electrochemical deposition at any point on a substrate. Localized electrochemical deposition encompasses traditional localized electrochemical deposition technology (LECD) [23,24,25], electrochemical printing (EcP) [26], meniscus confined electrodeposition (MCED) [27,28,29], and LECD micro-AM technology (LECD-µAM) [3]. The structures generated using LECDs typically exhibit be relatively high porosity, with structural dimensions depending on the size of micro and conducting tips. The adjustment of the range and current density of microjets presents a complex and challenging task in the deposition process of EcP technology. As a result, difficulties are encountered in the achievement of precise control. The microstructures fabricated via MCED technology display a superior surface quality and attain deposition sizes down to the nanometer scale. In the application of this technology, multiple factors, including the relatively slow deposition rate and regression rate of the micropipette, are controlled to prevent liquid bend fracture. In addition, the growth state of nanowires in the deposition structure must be regulated. Furthermore, to address the limitations inherent in conventional electrochemical machining technology, researchers have explored the viability of composite electrochemical machining technology. Examples include magnetic electrochemical finishing [30, 31], ultrasonically assisted electrochemical machining [32], and laser and electrochemical machining [33]. However, the composite electrochemical machining technology used for the preparation of microstructures with high efficiency, precision, and quality still needs to be explored.
The present study employed LECD-µAM technology in conjunction with an automated feedback mechanism. The deflection of the hollow atomic force microscopy (AFM) cantilever probe was converted into an electrical signal. This step was followed by the subsequent transmission of the signal to the control system and its feedback to the cantilever probe for displacement. Furthermore, the extrusion pressure propelled the electrolyte solution and induced forced convection during the deposition process, which ensured a constant micron deposition rate and high-quality deposition. The design of a CSV file of the matching space point cloud for layer-by-layer deposition printing offers distinct advantages in the manufacture of intricate 3D components. The electrolyte flow in the cantilever probe was simulated, and the effect of extrusion pressure on the deposition microstructure was investigated through experimental analysis. The technology can be implemented at room temperatures [34], with minimal environmental requirements and low cost, while providing excellent deposition surface quality. Furthermore, the proposed technology exhibits potential use in the fabrication of complex 3D microstructure arrays.
2 Experimental Sections
2.1 Experiment Preparation
The cathode substrate was prepared using a 0.5 mm-thick N-type single-crystal silicon wafer, with a 15 nm-thick layer of chromium plated as a bonding layer via magnetron sputtering technology. Then, another layer of copper with a thickness of 100 nm was added. Finally, the substrate was cut into a square (15 × 15 mm2) using a diamond dicing machine (Beijing Tebowande Technology Co., Ltd.), as shown in Fig. 1.
Prior to electrochemical deposition, the cathode substrate was surface cleaned through the following steps. First, the cathode substrate was initially immersed in a beaker containing an acetone solution and ultrasonically cleaned for 3 min. This step is crucial for the effective removal of persistent organic matter. Subsequently, the clamped cathode substrate was sequentially rinsed with isopropyl alcohol and deionized water to eliminate any remaining impurities. Finally, substrate drying under nitrogen gas was performed for electrochemical deposition.
2.2 Experimental Flow of LECD-µAM Technology
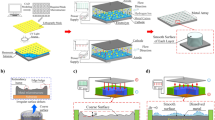
Figure 2 illustrates the manufacturing principle of the LECD-µAM technology. The technical equipment includes an atomic force probe system, a pressure control system, an electrolytic cell system, a feedback system, a micromechanical system, and a servo motor macro system [35].
The electrolytic cell system depicted in Fig. 2 assumes a three-electrode configuration, with annular graphite, Ag/AgCl line, and the aforementioned copper-plated silicon wafer serving as the anode, reference electrode, and cathode, respectively. For a guaranteed conducive electric environment for deposition, the cathode was positioned on an insulating plate at the base of the electrolytic cell and connected to the lower portion of the graphite electrode. Furthermore, an acidic support solution (54 mM H2SO4, 0.5 mM HCl) was introduced into the electrolytic cell system. The system’s moving platform facilitated the overall cell control. A slender gas conduit, which enabled software-driven collaborative control for the localized electrolyte outflow (0.5 M CuSO4, 51 mM H2SO4, 0.48 mM HCl), connected the pressure control system to the atomic force probe system. As a result, localized electrochemical functionality was achieved. The pressure controller was supervised via software to control the extrusion pressure precisely. Meanwhile, the driven electrolyte was allowed to flow from the microhole to the support solution through the hollow probe. During the localized electrochemical deposition process, once the first deposited voxel came into contact with the probe and its deflection surpassed a predetermined threshold, the optical feedback system converted the force signal into an electrical signal and provided feedback to the control system. As a result, the probe proceeded to the subsequent position for deposition. Monitoring of the deposition process was accomplished using high-powered overhead cameras and equipment.
2.3 Characterization
The experiments in this study were conducted in a CERES system (Exaddon, Glattbrugg, Switzerland), which features a micropipette (Exaddon) and a microfluidic delivery system for printing. The surface morphology of the substrate was also characterized via tungsten-filament scanning electron microscopy (SEM, ZEISS, EVO25). The chemical composition and elemental distribution of the surfaces were analyzed through energy-dispersive Xray spectroscopy (EDS) (X-Max, Oxford). Comparison and analysis of the size characteristics of the microstructure were performed after the depositions under various parameters.
2.4 Test Environment
The test condition involved the use of a microchannel AFM cantilever probe immersed in a three-electrode electrochemical containing a silver reference electrode and a platinum pair electrode for the desired localized electrodeposition under the tip. A pressure controller was used for the precise control of the outflow of metal salt solution from the cantilever probe tip. We mainly explored the effect of extrusion pressure, which drives the flow of the electrolyte solution, on deposition results. Specifically, 0.5 mol/L copper sulfate solution was used as the electrolyte at an extrusion pressure range of 20–160 mbar and an interval of 20 mbar while maintaining an interelectrode voltage of U = − 0.48 V.
3 Results and Discussion
3.1 Simulation Model Building
The local electrolyte transport in the pressure control system and atomic force probe system is primarily facilitated by a micropump, microvalve, and microfluidic channel. The atomic force probe comprised silicon and silicon carbide components, including a hollow microcantilever and a pyramid tip. The atomic force probe’s microcantilever had a total length and width of 200 and 40 μm, respectively, and the diameter of the pyramid tip was 300 nm. The hollow cantilever and atomic force probe equipped with the pyramid tip formed the microfluidic channel. The tail end of the hollow cantilever was connected to the microfluidic chip, which in turn was connected to the lower end of the liquid storage tank storing the electrolyte. The upper end of the liquid storage tank was sealed and connected. The pressure execution part was connected to the tail of the probe, where the pulse pressure was controlled using a pressure controller.
As metal ions only occupy the electrolyte inside the probe, the electrolyte’s flow performance is important. Consequently, the shape and size of deposits can be adjusted within a certain range through modification of the pressure of the extruded metal salt solution without probe alteration. We simplified the technology simulation model of fixed-domain electrochemical deposition micro-AM (Fig. 3). The gray area in Fig. 3 represents the condition with the complete present of the internal electrolyte solution of the probe. The left end face of the electrolyte solution served as the entrance, and the pyramid tip of the atomic force probe at the lower right functioned as the bottom exit. Electrolyte generation occurred through the application of pressure to copper sulfate within an electrolytic cell, followed by deposition in a three-electrode system. To simulate the flow phenomenon of the electrolyte, we established a corresponding extrusion pressure and analyzed its influence on the deposition structure through experimentation. The simplified Hagen–Poiseuille law, which is further simplified for the laminar flow regime, was adopted.
3.2 Simulation Analysis
The electrolyte solution, as a carrier of sedimentary metal copper ions, considerably affected the movement of copper ions. Studies have confirmed that the introduction of forced pairing can accelerate the spreading rate and increase the sedimentation rate [17]. This technology includes the entry of the electrolyte into the electrolytic cell under extrusion pressure. Forced convection during the sedimentation process ensures the timely replenishment of deposited metal ions without the issue of missing ions, unlike the traditional electrochemical deposition methods. Specifically, the highest velocity and pressure of Cu2+ were observed during the sedimentation process. At the pressure of 20 mbar, 2.5 s (Fig. 4), the velocity and pressure distribution of the probe port is more intuitive.
The simulation results reveal the highest flow velocity and pressure of the copper sulfate solution at the probe tip. Localized electrochemical deposition reactions received sufficient reactive copper ions and deposited at the same location, allowing each deposition to cover the previously deposited voxels. At a certain pressure, the absence of void phenomena led to the formation of a structure with a superior tissue density.
3.3 Experiment
The extrusion pressure, which drove the flow of the electrolyte solution, influenced the liquid-phase–mass-transfer process and consequently the deposition outcome [20]. In this study, copper micropillars with a height of 25 µm were deposited at an interelectrode voltage of U = − 0.50 V. The extrusion pressure ranged from 60 to 160 mbar with intervals of 20 mbar.
As displayed in Fig. 5, as the extrusion pressure increased, the diameter of the electrodeposited micropillar also increased. Simultaneously, within this pressure range, the surface quality of the sedimentary body improved with the increase in pressure. This condition led to a considerable reduction in surface defects of the copper column and an overall improvement in surface quality.
The rough side walls presented in Fig. 5 exhibited specific printing details. Our treatment method involves layer-by-layer printing, with each layer being interconnected. Notably, at the top of the figure, a volcanic shape was obtained for each copper micropillars. The upper edge of the cylindrical body exhibits a rising and decreasing trend due to pressure factors at the probe mouth. During the actual printing process, the upper and lower parts bonded together and formed cohesive sediment during printing. Consequently, this sediment’s surface exhibited a more pronounced texture. This texture can be minimized by increasing the electrolyte extrusion pressure to improve the outflow. A smoother surface can be achieved by increasing the pressure.
Simultaneously, as illustrated in Fig. 6, within this range, the diameter of the micropillar of electricity sinks increased with the escalation in extrusion pressure. Consequently, a gradual enlargement of unexpected sedimentary particles occurred at the bottom. Furthermore, the deposition length showed a gradual decline, and at the pressure ranges from 60 to 100 mbar, a rapid reduction in time was utilized. Subsequently, the sedimentation rate increased progressively. However, within the specific range of extrusion pressure, the deposition rate can reach up to 0.961 µm/s, and its body deposition rate exhibited a positive correlation with the increased extrusion pressure.
Experiments on copper microcolumn deposition were conducted at a deposition height of 15–45 µm and an interval of 10 µm. The extrusion pressure was varied between 60 and 160 mbar, with an interval of 20 mbar, and the electrode voltage remained constant at − 0.48 V. Figure 7a presents the SEM images of the copper microcolumns obtained from the experiments.
To gain further insights into the surface chemical composition of the deposited structure, we used X-ray spectroscopy in the analysis of copper micropillars (Fig. 7b). According to the scanning analysis results, the deposited structure contained 0.5% oxygen on its surface, and this finding can be attributed to the formation of some oxides due to surface exposure to oxygen. However, a copper content as high as 99.5% indicates an exceptionally pure elemental composition. Furthermore, the surface showed no other elements that originated from the electrolyte solution were detected, which highlights the reliability of the deposition environment.
The structure fabricated using this technology was solid and showed no hollow phenomena. In addition, each deposition covered completely the deposited voxels to achieve seamless connections between them. This deposition technique possesses a unique self-healing capability, enabling the fabrication of structures with high tissue density. Subsequently, experiments on copper micropillar array deposition were conducted. The distribution interval of the array was designed, and the deposition path was analyzed and planned. Figure 8 depicts the specific route experimental route. To elucidate the process further, we created arrays consisting of microcolumns arranged in a 5 × 5 configuration. Figure 9 illustrates the copper micropillars printed at 160 mbar, a height of 35 microns, and a spacing of 3 microns between each microcolumn. Each duty cycle in the array had a total printing time of 576 s, which resulted in the printing of 40,470 voxels. Minimal variation was observed in each microcolumn, which led to an overall excellent surface quality outcome. These results highlight the remarkable developmental potential of this printing process.
The localized electrochemical deposition micro-AM technology offers crucial advantages for the production of complex structural parts. Figure 10 showcases the intricate microstructures that have been successfully fabricated using the proposed technology. Complex microstructures demonstrated versatility and precision, which are achievable through localized electrochemical deposition. Thus, the findings highlight the potential for the manufacture of intricate and sophisticated components. Figure 10a reveals the microstructural array surface of this structure under pressures from 40 to 160 mbar. Figure 10b and c reveal the deposition at pressures of 160 and 40 mbar, respectively. These results verify the effect of pressure on the morphology of sedimentary bodies.
Then, we used LECD-µAM technology to prepare a square-top array structure with an array area of 600 × 600 μm2 (Fig. 11). This technology features manufacturing consistency and feasibility in the production of large-area arrays of microadditive micron-scale structures and has great advantages.
4 Conclusion
The miniaturized parts with AM of metal microstructure arrays find extensive applications as fundamental carriers in aerospace, microelectronics packaging, microsensors, and biomedicine. Conventional manufacturing methods, which are used for metal and alloy processing, exhibit intricacy, limited material utilization rate, and challenges in intricate structural formations. The electrochemical deposition AM technology offers a concise process route, convenient design capabilities, cost-effectiveness, and environmental sustainability, which resulted in remarkable advancements. In this study, LECD-µAM technology was used for the layer-by-layer deposition of microstructures without the application of supporting materials, such as masks, and with an automatic feedback mechanism. The specific conclusions include the following:
-
(1)
Through simulation of the pressure-driven electrolyte solution flow state, we obtained the pressure distribution at the probe outlet and investigated its effect on the surface of the deposition structure via experimental analysis. The diameter of the deposited microcolumn decreased within a specific range was the electrolyte extrusion pressure was reduced. The extrusion pressure and time can be dynamically adjusted in real time to attain the regulation of the deposition structure’s diameter. The sedimentary body in the combined state showed consistency with the outer surface of the monomer microstructure, and its sedimentary quality was good. This method allows real-time adjustment of the diameter of the deposited structure, which is highly important for the improved quality of metal microstructure manufacturing.
-
(2)
Prior to the deposition of the metal microstructure array, the deposition parameters were optimized based on the preparation of monomer microstructures. The deposition route was designed, the spacing of the microstructure array was adjusted, and the deposition path was planned. These properties can prevent collisions between the deposition structure and reduce collisions between the deposition structure and the probe, which enables the fabrication of an array of metal microstructures.
-
(3)
We analyzed the chemical composition of the sedimentary structure and observed the exceptional deposition quality, with a copper content of up to 99.5%, confirming the capability to fabricate high-purity metals via LED-µAM. Through high-quality deposition, micron-scale intricate 3D array metal microstructure printing can be achieved at the. This technology offers advantages, such as reduced dependency on the environment and superior surface quality of sedimentation. Furthermore, the 3D metal microstructure array holds immense potential in various fields, including micro- and nano-processing, biomedicine, and optoelectronics. The method features convenient preparation, is cost-effective, has controllable structure formation, and thus offers important prospects for innovation and development.
Availability of Data and Materials
The authors declare that all data supporting the findings of this study are available within the article.
References
Li X, Ming P, Ao S, Wang W (2022) Review of additive electrochemical micro-manufacturing technology. Int J Mach Tools Manuf 173:103848
Hirt L, Reiser A, Spolenak R, Zambelli T (2017) Additive manufacturing of metal structures at the micrometer scale. Adv Mater 29(17):1604211
Xu J, Ren W, Lian Z, Yu P, Yu H (2020) A review: development of the maskless localized electrochemical deposition technology. Int J Adv Manuf Technol 110:1731–1757
He M, Zhao Y, Wang B, Xi Q, Zhou J, Liang Z (2015) 3D printing fabrication of amorphous thermoelectric materials with ultralow thermal conductivity. Small 11(44):5889–5894
Jung HY, Choi SJ, Prashanth KG, Stoica M, Scudino S, Yi S, Eckert J (2015) Fabrication of Fe-based bulk metallic glass by selective laser melting: a parameter study. Mater Des 86:703–708
Wang P, Sin WJ, Nai MLS, Wei J (2017) Effects of processing parameters on surface roughness of additive manufactured Ti-6Al-4V via electron beam melting. Materials 10(10):1121
Körner C (2016) Additive manufacturing of metallic components by selective electron beam melting—a review. Int Mater Rev 61(5):361–377
Kolamroudi MK, Asmael M, Ilkan M, Kordani N (2021) Developments on electron beam melting (EBM) of Ti–6Al–4V: a review. Trans Indian Inst Met 74:783–790
Safdar A, He HZ, Wei LY, Snis A, Chavez de Paz LE (2012) Effect of process parameters settings and thickness on surface roughness of EBM produced Ti-6Al-4V. Rapid Prototyp J 18(5):401–408
Regenfuss P, Streek A, Hartwig L, Klötzer S, Brabant T, Horn M, Exner H (2007) Principles of laser micro sintering. Rapid Prototyp J 13(4):204–212
Exner H, Horn M, Streek A, Ullmann F, Hartwig L, Regenfuß P, Ebert R (2008) Laser micro sintering: a new method to generate metal and ceramic parts of high resolution with sub-micrometer powder. Virtual Phys Prototyp 3(1):3–11
Regenfuss P, Hartwig L, Klötzer S, Ebert R, Brabant T, Petsch T, Exner H (2005) Industrial freeform generation of microtools by laser micro sintering. Rapid Prototyp J 11(1):18–25
McLouth TD, Witkin DB, Lohser JR, Sitzman SD, Adams PM, Lingley ZR, Zaldivar RJ (2021) Temperature and strain-rate dependence of the elevated temperature ductility of Inconel 718 prepared by selective laser melting. Mater Sci Eng A 824:141814
Turalıoğlu K, Taftalı M, Yetim F (2021) Determining the tribological behavior of 316L stainless steel with lubricating micro-channels produced by the selective laser melting (SLM) method. Ind Lubr Tribol 73(5):700–707
Navi NU, Rosen BA, Sabatani E, Tenenbaum J, Tiferet E, Eliaz N (2021) Thermal decomposition of titanium hydrides in electrochemically hydrogenated electron beam melting (EBM) and wrought Ti–6Al–4V alloys using in situ high-temperature X-ray diffraction. Int J Hydrog Energy 46(59):30423–30432
Zou Z, Xu J, Ren W (2023) Novel hollow re-entrant structures improving hydrophobicity of metal surfaces. Nanomanuf Metrol 6(1):12
Zhang J, Li Y, Cao K, Chen R (2022) Advances in atomic layer deposition. Nanomanuf Metrol 5(3):191–208
Wang Y, Yi F, Zhang T, Liu J, Wang B, Zhou Y (2017) Design and property study of micro-slot optics. Opt Commun 386:14–21
Bai WQ, Li YG, Yang CS, Liu JQ, He DN, Sugiyama S (2012) Fabrication of metal micro needle array by LIGA process. Adv Mater Res 418:1911–1914
Becker E, Ehrfeld W, Hagmann P, Maner A, Münchmeyer D (1986) Fabrication of microstructures with high aspect ratios and great structural heights by synchrotron radiation lithography, galvanoforming, and plastic moulding (LIGA process). Microelectron Eng 4(1):35–56
Alper SE, Ocak IE, Akin T (2007) Ultrathick and high-aspect-ratio nickel microgyroscope using EFAB multilayer additive electroforming. J Microelectromech Syst 16(5):1025–1035
Cohen A (2014) MICA freeform vs selective laser melting. Online: http://www.microfabrica.com/downloads/MIC-WhitePaper-2014.pdf
Thornell G, Jansson A, Johansson SA (1999) Direct writing of nickel by electrodeposition from various electrolytes. In: Device and process technologies for MEMS and microelectronics. SPIE, vol 3892, pp 166–175
Sundaram M, Kamaraj AB, Lillie G (2018) Experimental study of localized electrochemical deposition of Ni-Cu alloy using a moving anode. Procedia CIRP 68:227–231
Park J, Kim H (2010) High aspect ratio 3-D electroplating assisted by localized laser irradiation. In: 2010 IEEE 5th international conference on nano/micro engineered and molecular systems. IEEE, pp 1120–1123
Chen YL, Wang Y, Wang Y, Ju BF (2021) Meniscus-confined electrodeposition of metallic microstructures with in-process monitoring of surface qualities. Precis Eng 70:34–43
Hengsteler J, Mandal B, van Nisselroy C, Lau GP, Schlotter T, Zambelli T, Momotenko D (2021) Bringing electrochemical three-dimensional printing to the nanoscale. Nano Lett 21(21):9093–9101
Liao X, Zhuang J, Deng Y, Wang Z, Zheng Q, Cai Y, Lu X (2020) A closed-loop constant deposition current control method based on MCED circuit model. AIP Adv 10(4)
Yeo SH, Choo JH, Sim KHA (2002) On the effects of ultrasonic vibrations on localized electrochemical deposition. J Micromech Microeng 12(3):271
Jia JL, Fan ZJ (2011) Research on higher frequency, short pulses and assisted magnetic field electrochemical machining. Adv Mater Res 189:3162–3165
Ruszaj A, Skoczypiec S, Czekaj J, Miller T, Dziedzic J (2007) Surface micro and nanofinishing using pulse electrochemical machining process assisted by electrode ultrasonic vibrations. In: Proceedings of the 15th international symposium on electromachining–ISEM XV, pp 23–27
Rao BT, Kaul R, Tiwari P, Nath AK (2005) Inert gas cutting of titanium sheet with pulsed mode CO2 laser. Opt Lasers Eng 43(12):1330–1348
DeSilva AK, Pajak PT, Harrison DK, McGeough JA (2004) Modelling and experimental investigation of laser assisted jet electrochemical machining. CIRP Ann 53(1):179–182
Ren W, Xu J, Lian Z, Sun X, Xu Z, Yu H (2021) Localized electrodeposition micro additive manufacturing of pure copper microstructures. Int J Extreme Manuf 4(1):015101
Zou Z, Xu J, Ren W, Wang M, Xu Z, Wang X, Yu H (2023) Additives induced localized electrodeposition manufacturing of high quality copper micro-rectangular solid structures. J Mater Process Technol 321:118152
Acknowledgements
This work was supported by the National Key R&D Program of China (no. 2022YFB4600202); and Jilin Province Science and Technology Development Key Research and Development project [No.20230201093GX].
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, M., Xu, J., Ren, W. et al. Fabrication of Microstructure Arrays via Localized Electrochemical Deposition. Nanomanuf Metrol 7, 15 (2024). https://doi.org/10.1007/s41871-024-00236-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41871-024-00236-7