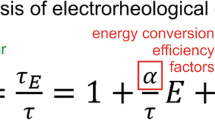
Abstract
In this paper, the variation of suspension stability under the electric field was studied. Ceramic suspensions such as YSZ/acetyl acetone, YSZ/1propanol, and SnO2/ethanol were analyzed by applying different voltages. The novel turbidimetry technique was employed to illustrate the stability changes versus electrophoretic deposition (EPD) time. Macro-photography was employed to study the colloidal stability before and after EPD. Also, the weight of the deposited particles was measured. Particles in the suspension were sediment after applying voltage, making the suspension transparent in our deposition cell, especially at 100 V. The electrical conductivity of suspension increased by applying the electric field, showing a peak at 100 V. The suspension conductivity measurements also revealed a peak at 100 V. The occurred transparency became more in-depth over time. As suspension stability is an essential factor for a successful EPD process, the electric field-assisted agglomeration of particles should be considered in tests to reach repeatable results. Our research showed that despite the use of high voltage in routine EPD research, the instability of suspensions and its effect on the results of the coating should be considered.
Graphical abstract












Similar content being viewed by others
References
Mori, Y., Nobuzane, Y., Nishimura, K., Yamada, K., Tsuchiya, K.: Thin film structure of titania nanoparticles prepared by electrophoretic deposition. Chem. Eng. Trans. 57, 1507–1512 (2017). https://doi.org/10.3303/CET1757252
Besra, L., Liu, L.: A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater Sci. 52(1), 1–61 (2007). https://doi.org/10.1016/j.pmatsci.2006.07.001
Hamaker, H.: Formation of a deposit by electrophoresis. Trans. Faraday Soc. 35, 279–287 (1940). https://doi.org/10.1039/TF9403500279
Sarkar, P., Nicholson, P.S.: Electrophoretic deposition (EPD): mechanisms, kinetics, and application to ceramics. J. Am. Ceram. Soc. 79(8), 1987–2002 (1996). https://doi.org/10.1111/j.1151-2916.1996.tb08929.x
Besra, L., Pravanjan, S., Sarama, B., Bimal, S.: Electrophoretic deposition of alumina on stainless steel from non-aqueous suspension. J. Mater. Sci. 42(14), 5714–5721 (2007). https://doi.org/10.1007/s10853-006-0757-5
Mohanty, G., Besra, L., Sarama, B., Bimal, S.: Optimization of electrophoretic deposition of alumina onto steel substrates from its suspension in iso-propanol using statistical design of experiments. Mater. Res. Bull. 43(7), 1814–1828 (2008). https://doi.org/10.1016/j.materresbull.2007.07.014
Moreno, R., Ferrari, B.: Effect of the slurry properties on the homogeneity of alumina deposits obtained by aqueous electrophoretic deposition. Mater. Res. Bull. 35(6), 887–897 (2000). https://doi.org/10.1016/S0025-5408(00)00288-9
Sadeghi, A.A., Ebadzadeh, T., Raissi, B., Ghashghaie, S., Fateminia, S.M.A.: Application of the multi-step EPD technique to fabricate thick TiO2 layers: effect of organic medium viscosity on the layer microstructure. J. Phys. Chem. B 117(6), 1731–1737 (2013). https://doi.org/10.1021/jp306976p
Yavuz, M., Cabuk, M.: Electrorheological properties of pumice/silicone oil suspension. J. Mater. Sci. 42(6), 2132–2137 (2007). https://doi.org/10.1007/s10853-006-1296-9
Lemaire, E., Lobry, L., Pannacci, N., Peters, F.: Viscosity of an electro-rheological suspension with internal rotations. J. Rheol. 52(3), 769–783 (2008). https://doi.org/10.1122/1.2903546
Ahualli, S., Gonzalez, M.A., Delgado, A.V., Jimenez, M.L.: Dynamic electrophoretic mobility and electric permittivity of concentrated suspensions of plate-like gibbsite particles. J. Colloid Interface Sci. 502, 112–121 (2017). https://doi.org/10.1016/j.jcis.2017.04.072
Shikinaka, K., Kimura, H.: Reversible viscosity change of nanotubular colloidal aqueous suspensions responding to an electric field. Colloids Surf., A 459, 1–3 (2014). https://doi.org/10.1016/j.colsurfa.2014.06.035
Cañizares, P., Martinez, F., Jimenez, C., Lobato, J., Rodrigo, M.A.: Coagulation and electrocoagulation of wastes polluted with colloids. Sep. Sci. Technol. 42(10), 2157–2175 (2007). https://doi.org/10.1080/01496390701446530
Qiao, X., Sun, A., Wang, C., Chu, C., Ma, S., Tang, X., Guo, J., Xu, G.: Electric field induced structural color changes of highly monodisperse hollow Fe3O4@ C colloidal suspensions. Colloids Surf., A 498, 74–80 (2016). https://doi.org/10.1016/j.colsurfa.2016.03.027
B. Raissi, R. Riahifar, M. Sahba Yaghmaee, F. Taati-Asil, A. Aghaei, S. Chatrnoor, A.H. Taghadossi, R. Irankhah, and M. Karimi. DC-EPD of nanoceramic particles accelerated via anodic dissolution in organic media. Int. J. Mater. Res. 109(4): 356–361 (2018). https://doi.org/10.3139/146.111603
Mollah, M.Y.A., Schennach, R., Parga, J.R., Cocke, D.: Electrocoagulation (EC)—science and applications. J. Hazard. Mater. 84(1), 29–41 (2001). https://doi.org/10.1016/s0304-3894(01)00176-5
Peng, Z., Doroodchi, E., Evans, G.: DEM simulation of aggregation of suspended nanoparticles. Powder Technol. 204(1), 91–102 (2010). https://doi.org/10.1016/j.powtec.2010.07.023
Farbod, M., Tadavani, S.K., Kiasat, A.: Surface oxidation and effect of electric field on dispersion and colloids stability of multiwalled carbon nanotubes. Colloids Surf., A 384(1), 685–690 (2011). https://doi.org/10.1016/j.colsurfa.2011.05.041
S.S. Ahmadi, R. Riahifar, B. Raissi, M. Sahba Yaghmaee, H.S. Cornelis Metselaar M. Javaheri. Electrophoretic deposition of MWCNT on a PTFE layer for making working Electrode of oxygen sensor. J. Electrochem. Soc. 164(12) B506-B512 (2017). https://doi.org/10.1149/2.0461712jes.
A.A. Sadeghi Ghazvini, E. Taheri-Nassaj, B. Raissi, R. Riahifar, and M. Sahba Yaghmaee. Effect of polyethylenimine addition and washing on stability and electrophoretic deposition of Co3O4 nanoparticles. J. Am. Ceram. Soc. 101(2) 553–561 (2018). https://doi.org/10.1111/jace.15244.
Talebi, T., Raissi, B., Haji, M., Maghsoudipour, A.: The role of electrical conductivity of substrate on the YSZ film formed by EPD for solid oxide fuel cell applications. Int J Hydro Energy. 35(17), 9405–9410 (2010). https://doi.org/10.1016/j.ijhydene.2010.04.011
Talebi, T., Haji, M., Raissi, B., Maghsoudipour, A.: YSZ electrolyte coating on NiO–YSZ composite by electrophoretic deposition for solid oxide fuel cells (SOFCs). Int. J. Hydrogen Energy 35(17), 9455–9459 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.021
Talebi, T., Raissi, B., Maghsoudipour, A.: The role of addition of water to non-aqueous suspensions in electrophoretically deposited YSZ films for SOFCs. Int J Hydro Energy 35(17), 9434–9439 (2010). https://doi.org/10.1016/j.ijhydene.2009.12.152
Talebi, T., Haji, M., Raissi, B.: Effect of sintering temperature on the microstructure, roughness and electrochemical impedance of electrophoretically deposited YSZ electrolyte for SOFCs. Int. J. Hydrogen Energy 35(17), 9420–9426 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.079
T. Talebi, M. Hassan Sarrafi, M. Haji, B. Raissi, and A. Maghsoudipour. Investigation on microstructures of NiO–YSZ composite and Ni–YSZ cermet for SOFCs. Int. J. Hydro. Energy. 35(17) 9440–9447 (2010). https://doi.org/10.1016/j.ijhydene.2010.04.156
Wiśniewska, M., Urban, T., Nosal-Wiercinska, A., Zarko, V.I., Gun’ko, V.M.: Comparison of stability properties of poly (acrylic acid) adsorbed on the surface of silica, alumina and mixed silica-alumina nanoparticles—application of turbidimetry method. Cent. Eur. J. Chem. 12(4), 476–479 (2014). https://doi.org/10.2478/s11532-013-0401-6
Bernardo, F.O.C., Silva, J.M., Canevarolo, S.V.: Dispersed particle size characterization by in-line turbidimetry during polymer extrusion. Polym. Testing 70, 449–457 (2018). https://doi.org/10.1016/j.polymertesting.2018.08.005
Sa’adati, H., Raissi, B., Riahifar, R., Sahbayaghmaee, M.: How preparation of suspensions affects the electrophoretic deposition phenomenon. J. Eur. Ceram. Soc. 36(2), 299–305 (2016). https://doi.org/10.1016/j.jeurceramsoc.2015.09.005
N. Abavi Torghabeh, B. Raissi, R. Riahifar, M. Sahbayaghmaee, and Z, Minaei Bidgoli. Investigation of the flocculation and sedimentation of TiO2 nanoparticles in different alcoholic environments through turbidity measurements. J. Compos. Compd. 3(8) 159–163 (2021). https://doi.org/10.52547/jcc.3.3.2
Kollath, V.O., Chen, Q., Closset, R., Luyten, J., Traina, K., Mullens, S., Boccaccini, A.R., Cloots, R.: AC vs. DC electrophoretic deposition of hydroxyapatite on titanium. J Eur. Ceram. Soc. 33(13–14), 2715–2721 (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.04.030
Lobry, L., Lemaire, E.: Viscosity decrease induced by a DC electric field in a suspension. J. Electrostat. 47(1–2), 61–69 (1999). https://doi.org/10.1016/S0304-3886(99)00024-8
Pannacci, N., Lobry, L., Lemaire, E.: How insulating particles increase the conductivity of a suspension. Phys. Rev. Lett. 99(9), 094503 (2007). https://doi.org/10.1103/PhysRevLett.99.094503
Biesheuvel, P.M., Verweij, H.: Theory of cast formation in electrophoretic deposition. J. Am. Ceram. Soc. 82(6), 1451–1455 (1999). https://doi.org/10.1111/j.1151-2916.1999.tb01939.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torghabeh, N.A., Riahifar, R., Raissi, B. et al. Effect of applying electric field on suspension stability during electrophoretic deposition of ceramic particles in nonaqueous media: a case study. J Aust Ceram Soc 58, 735–745 (2022). https://doi.org/10.1007/s41779-021-00693-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-021-00693-z