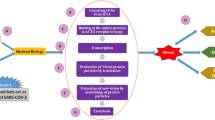
Abstract
Interface with animals has been responsible for the occurrence of a major proportion of human diseases for the past several decades. Recent outbreaks of respiratory, haemorrhagic, encephalitic, arthropod-borne and other viral diseases have underlined the role of animals in the transmission of pathogens to humans. The on-going coronavirus disease-2019 (COVID-19) pandemic is one among them and is thought to have originated from bats and jumped to humans through an intermediate animal host. Indeed, the aetiology, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can infect and cause disease in cats, ferrets and minks, as well as be transmitted from one animal to another. The seriousness of the pandemic along with the zoonotic origin of the virus has been a red alert on the critical need for collaboration and cooperation among human and animal health professionals, as well as stakeholders from various other disciplines that study planetary health parameters and the well-being of the biosphere. It is therefore imminent that One Health principles are applied across the board for human infectious diseases so that we can be better prepared for future zoonotic disease outbreaks and pandemics.
Similar content being viewed by others
1 Introduction
The COVID-19 pandemic is a stark reminder that we live in a time where pathogens spread faster than we can track them. The incidence of other diseases in humans such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS),1 H1N1 pandemic influenza,2 H5N1 avian influenza,3 Ebola haemorrhagic fever,4 etc. in just the last two to three decades has also highlighted that globalization and animal–human interface are major factors in the transmission of pathogens from wild and domestic animals to humans.
Recent advances, especially in genomics, have spurned the understanding of diseases in terms of their aetiology, origin, identification of index case, routes of spread and potential transmission, evolution of the pathogen, minutest details of host–pathogen interaction and pathogenesis, and vaccine and/or drug discovery, facilitating disease mitigation strategies. At least one new infectious disease is thought to emerge or re-emerge every year.5,6 Between 1940 and 2004, there were 335 emerging infectious diseases, about 60% of them originating from animals, with not much change in decade-wise proportion of zoonotic diseases.7 About three-fourths of all emerging and re-emerging human diseases can be traced back to animals, particularly wild animals,7 and it has been propounded, probably rather radically, that every species of Kingdom Animalia may be a potential carrier of human pathogens.8
2 SARS-CoV-2 and Its Origin
The SARS-CoV-2 belongs to the family Coronaviridae and has one of the largest single-stranded RNA genomes (29.9 kilo bases) for a virus. The major structural proteins encoded by SARS-CoV-2 are spike (S), nucleoprotein, envelope and membrane proteins. Among these structural proteins, the S protein is critical for the receptor-mediated entry of the virus into susceptible cells. The receptor binding domain (RBD) of the S protein binds the cellular receptor, the angiotensin converting enzyme-2 (ACE2),9 facilitating entry of the virion particle. Further, transmembrane protease serine 2 (TMPRSS2) helps in the priming of the S protein, where the S protein is cleaved so as to allow the fusion of viral envelope with cellular membrane.9 In vitro, ACE2 from as many as 44 mammals has been shown to bind to the RBD.10,11 On the other hand, modelling studies have returned supporting as well as conflicting results on the interaction between S and ACE2.12,13,14,15 Together, these studies only suggest interactions while receptor expression and other host factors may dictate infection, replication and pathogenesis as well as transmission to other hosts.
The first cases of COVID-19 are thought to have originated from live animal markets in Wuhan, China. Analysis of the genomes of SARS-CoV-2 has suggested that the virus may have originated from the Asian bats belonging to the Rhinolopus species16,17,18,19 and that the transmission to humans may have involved an intermediate host, possibly the pangolin.16,17,20,21,22,23 However, both the precise origin and the exact intermediate host are conjectures at best. On the other hand, higher sequence similarities of SARS-CoV-2 with bat coronavirus-RaTG13 (96.2%) and Guangdong Pangolin-CoV (92.4%)22,24 support the zoonotic origin of the virus. However, the SARS-CoV-2 has a unique “PRRA” motif at the junction of the S1 and S2 subunits of the S protein, which makes it distinct from RaTG13 or the Guangdong Pangolin-CoV. Further, the binding affinity of pangolin ACE2 receptor with RBD has been shown to be low.25 Recent genetic analysis has revealed that three viruses in bats from the limestone karstic terrain in North Laos, BANAL-52, BANAL-103 and BANAL-236 are very close to SARS-CoV-2 with only one or two mutations in the RBD and the absence of a cleavage site for the cellular enzyme furin. These viruses are therefore considered to be the closest match to SARS-CoV-2 in nature.26
3 Animal Models for SARS-CoV-2 Infection
Experimental reproduction of COVID-19 in mice requires adaptation of SARS-CoV-2 through serial passage27 as wild-type mice are resistant to infection.28 However, mouse adaptation also leads to changes in SARS-CoV-2, which is not ideal for challenge studies. When mutations relevant to mouse adaptation were introduced through reverse genetics, the resultant virus replicated in the upper and the lower respiratory tract of BALB/c mice when the virus was administered intranasally.29 The main reason for the species restriction appears to be the receptor, since SARS-CoV-2 not adapted to mice or adapted to grow in cell culture can efficiently infect transgenic mice expressing human ACE2 (hACE2),28,29,30,31 and such mice are considered suitable for challenge studies. Some transgenic mice such as HFH4-hACE2 mice express the receptor in the respiratory tract and the central nervous system, resulting in lethal encephalitis upon infection through the respiratory route.29,30,32 A sole study has also reported that when administered intragastrically, the virus could potentially be transmitted by the faecal–oral route33; however, the implications of this to transmission in humans are not clear, although SARS-CoV-2 causes gastroenteritis and is excreted in the faeces in humans, but faecal–oral transmission needs further evidence.34
Transient expression of hACE2 has also been employed for mouse infection studies. This method has the advantage of utilizing mice from diverse genetic backgrounds. Adenovirus or adeno-associated virus-mediated transduction has been used to express hACE2 in the respiratory tract of mice.35,36 When mice were transduced with human adenovirus 5 expressing hACE2 and subsequently infected with SARS-CoV-2 through intranasal or intranasal and intravenous route, only the peripheral and not the systemic route resulted in lung infection in mice.35 In mice transduced with adeno-associated virus 9 expressing hACE2, the transcriptome profile mimicked those in COVID-19 patients, in that interferon (IFN)-stimulated genes and inflammatory cytokines were up-regulated during SARS-CoV-2 infection. It was also observed that type I IFNs were responsible for pathological changes rather than controlling SARS-CoV-2 replication.36
Unlike mice, hamsters are susceptible to SARS-CoV-2 infection without the need for adaptation of the virus.30,37,38 Upon infection, hamsters lose weight initially but regain it by 14 days post-infection. Highest viral loads are detected in the nasal turbinates, lungs and trachea, facilitating the transmission of the virus to in-contact hamsters.37 Similar to humans, aerosol and droplet transmission between hamsters was higher with the D614G mutant of the virus.30
Two mustelids, which are used for commercial (minks) or experimental (ferrets) purposes, are highly susceptible to SARS-CoV-2. Minks undergo efficient upper and lower respiratory tract infection, with viral RNA being detected in the nasal turbinates, soft palates, tonsils, all lung lobes and submaxillary lymph nodes and infectious virus being detected in most of these samples. Severe pathological conditions are observed in infected minks with extensive and diffuse consolidation of the lungs. Nasal mucosa and submucosa of the vestibular, respiratory and olfactory regions show inflammatory infiltrates, epithelial degeneration and necrosis. Notably, the SARS-CoV-2-mediated respiratory system damage is similar to what is observed in severe human COVID-19 cases. Transmission of SARS-CoV-2 between minks through droplets has also been demonstrated.39
Ferrets are also suitable animal models. SARS-CoV-2-infected ferrets show infectious virus in the upper respiratory tract40 and virus is shed in saliva, urine and faeces.41 Infected ferrets transmit the virus through respiratory droplets to direct and indirect contact ferrets.42 Similar to ferrets, racoon dogs infected with SARS-CoV-2 shed the virus through nasal route, leading to infection of in-contact animals. However, the racoon dogs do not show any clinical signs.43
Experimentally, SARS-CoV-2 has also been shown to replicate in cats and be excreted in respiratory secretions, and infected cats can transmit the virus to co-housed cats.44 On the other hand, virus replication is poor in bank voles, cattle, dogs, pigs, chicken, ducks and tree shrews.40,45,46,47,48.
Experimental studies have also been carried out to study the SARS-CoV-2 infection in non-human primates (NHP). Infection produces acute respiratory distress and interstitial pneumonia in rhesus macaques, and severe pneumonia in baboons,49 but no clinical signs in cynomolgus monkeys.50 Irrespective of the clinical signs, SARS-CoV-2-infected NHPs show highest viral load in the upper and lower respiratory tract, indicating that NHP’s are a good model to study COVID-19 pathogenesis and protective efficacy of vaccines.
White-tailed deer produced subclinical infection upon intranasal administration of a SARS-CoV-2 isolate from a tiger, with the virus excreted in nasal secretions.51 Pregnant deer could transmit the virus through direct contact and vertically from doe to the foetus, with similar genome proportions in the tissues of the primary and in-contact animals and foetuses.52 Experimental infection of sheep with SARS-CoV-2 resulted in a mild infection and the viral RNA could be detected in nasal and oral swabs one day post-infection. However, the transmission of the virus from the infected sheep to naïve sheep was limited. Similar to white-tailed deer,52 when sheep were co-infected with two lineages of SARS-CoV-2, the ancestral lineage A appears to have less advantage over the α variant. However, these experiments have used 1:10 ratio of lineage A and α variant virus for the infection, making appropriate interpretation difficult.53
Details of the experimental infection of SARS-CoV-2 in animals are compiled in Table 1.
4 Natural Infection of Animals with SARS-CoV-2
Till date, 31 countries have reported SARS-CoV-2 infection in more than ten animal species (Table 2). While most of the infections are reported from pet animals, outbreaks have also been reported from organized mink farms, and zoo and wild animals.
Several observations have established that pet animals can be infected by SARS-CoV-2 (Table 2). Tests on 17 dogs and 8 cats from COVID-19 households in Hong Kong confirmed positivity in two dogs by molecular and serological assays, with virus being isolated in one case54,55; there has also been a press report of a dog showing mild respiratory symptoms and testing positive for SARS-CoV-2 in North Carolina, USA, but a cat and another dog from the same COVID-19 household tested negative.56
From a Belgian COVID-19 household, a cat showed respiratory and gastrointestinal symptoms and shed the virus in faeces and gastric fluid, but recovered.57 Two cats from two independent households in New York state also tested positive by reverse transcription—polymerase chain reaction (RT-PCR), one from a COVID-19 household and the other within an affected neighbourhood and allowed to go outdoors, with the latter reportedly showing respiratory symptoms.58,59 A cat from Hong Kong also showed positivity without symptoms.60 Further, 15 (14.7%) of 102 cats tested after the outbreak started in Wuhan were found to carry antibodies to SARS-CoV-2 by enzyme-linked immunosorbent assay (ELISA), and 11 samples showed neutralization of SARS-CoV-2 and no cross-reactivity with feline coronavirus; these samples had been obtained from stray cats, at a veterinary hospital and from animals owned by patients.61 Thus, it appears that while cats and other felids can be infected, the infection rate may be low, and a symptomatic outcome is rare. In support of this, a study has reported no infection of 9 cats and 12 dogs which were in close contact with COVID-19 patients in a veterinary campus in France,62 and only one asymptomatic (but positive by RT-PCR) cat among 12 dogs, 8 cats, 2 rabbits and one guinea pig from 17 confirmed COVID-19 households in Spain.63 In addition, a serological survey of preserved sera from 487 dogs and 87 cats from among 1914 samples from 35 species of animals from China showed no positivity, although the samples probably predated COVID-19.64 Studies on pets which belong to COVID-19 patients in Brazil also indicated that the pet animals were susceptible to SARS-CoV-2 infection. Nine out of 29 dogs and four out of 10 cats were seropositive for SARS-CoV-2. Partial genome sequence of SARS-CoV-2 was obtained from the samples, but no virus isolation was attempted.65 In most of the documented cases of SARS-CoV-2 infection in dogs and cats, the symptoms were variable; while most of the animals were asymptomatic, some of the animals had mild respiratory distress. Recent studies have also linked myocarditis as one possible outcome with SAR-CoV-2 B.1.1.7 variant infection of dogs and cats.66
Direct or indirect evidence of SARS-CoV-2 infection in pet animals during the COVID-19 pandemic exists from Germany, Italy, Peru, the USA, France and several other countries.65,67,68,69,70,71 In multiple cases, genome sequences of SARS-CoV-2 obtained from pet animals were similar to that of the circulating human strains. For example, the SARS-CoV-2 genome sequence from a cat had D614G mutation specific to the phylogenetic clade A2 observed in the French SARS-CoV-2 genome sequences.72 Studies in France also showed that the SARS-CoV-2 genome sequences from a pet dog and its COVID-19 affected owners were of B.1.160 lineage with 99–100% identity.73 The SARS-CoV-2 genome from a domestic cat was close to the human B.1.1.39 lineage in Switzerland.74 Similar observations have been made in Argentina, where the SARS-CoV-2 genome sequence from domestic cats belonged to the B.1.499 lineage, which circulated in humans in that region.75 In one instance, the SARS-CoV-2 genome sequence of the owner and the pet animals (dog and cat) from the same household were found to be identical and belonged to the B.1.575 lineage.76
In a seroprevalence study on samples collected from cats (2160 samples) from Germany, the UK, Italy and Spain, 96 samples (4.4%) were positive by virus neutralization test and 92 samples (4.3%) were positive by an ELISA for the RBD. Since the samples were collected for some other ailments and not for SARS-CoV-2 infection, the presence of antibodies against SARS-CoV-2 during the COVID-19 pandemic in the samples from cats indicates possible human-to-cat transmission.77 In Slovenia, a pet ferret of a COVID-19-affected owner was positive for SARS-CoV-2 by RT-PCR,78 and this supports the findings of the experimental studies where ferrets were documented to be highly susceptible for SARS-CoV-2 infection.40,41,42
5 SARS-CoV-2 Infection in Captive Animals
Apart from pet animals, SARS-CoV-2 can also infect captive and farm animals. Mild respiratory symptoms were reported in five tigers and three lions in the Bronx zoo, New York, supposedly from being in contact with a COVID-19 asymptomatic zookeeper; SARS-CoV-2 was confirmed by RT-PCR,79 the virus was isolated, and complete viral genome sequence was elucidated in the case of a Malayan tiger.80 Virus isolation was not attempted from other zoo animals. Interestingly, SARS-CoV-2 genome sequence from tigers and lions was of different genotypes, which indicates two independent sources of infection.
Other zoo and captive animals that can be affected include puma, mink (farmed and wild), lion, tiger, ferret, snow leopard, gorilla, otter, cougar, coatimundi, binturong, Canada lynx and hyena.78,81 While most of the animals showed mild symptoms of cough and respiratory distress with or without nasal discharge and recovered later, four snow leopards succumbed to SARS-CoV-2 infection at the Great Plains Zoo, South Dakota, USA.82,83
Fur industry across various countries has been affected severely due to the high susceptibility of minks to SARS-CoV-2. Netherlands reported the first outbreak of SARS-CoV-2 in a mink farm in April 2020. Since then, the disease has been reported from Denmark, the USA, France, Greece, Italy, Poland, Lithuania, Canada, Spain and Sweden. Minks are housed in confined spaces, and the infection could spread rapidly among them through droplets. Severity of respiratory sickness is pronounced in minks with high mortality and post-mortem findings of acute interstitial pneumonia.84 Considering the severity of the disease, Denmark, Spain, the Netherlands and France culled entire affected mink colonies and the Netherlands has banned mink farming permanently.
In the Netherlands, one mink farm worker was found positive for the virus and clear evidence of mink-to-mink transmission was established.85 Three out of 11 tested cats in one of the farms were also positive for anti-SARS-CoV-2 antibodies, but no viable virus could be detected,86 although the direction of transmission is not clear in this case. In one of the outbreaks, a probable mink-to-feral cat transmission was recorded.87 Mink-to-human transmission was also established based on the sequence data from the Netherlands,111 Denmark88 and the USA.89 SARS-CoV-2 virus isolates obtained from minks with Spike Y453F/D614G mutation have higher affinity to hACE2.90 Whole-genome sequencing of the SARS-CoV-2 from minks revealed 170 mutations, and the mink-specific mutations of SARS-CoV-2 were also found in 300 samples collected from humans indicating mink-to-human transmission.91 By contrast, the mink SARS-CoV-2 genome from Greece lacked the Y453F mutation in the S protein,92 suggesting differential adaptability or simply randomness in the emergence of the variants.
Recently, transmission of SARS-CoV-2 was recorded from Syrian hamsters to humans in a pet shop at Hong Kong. Further investigation revealed that Syrian hamsters from both pet shop (50%) and warehouse (58%) were positive for SARS-CoV-2 with RT-qPCR which resulted in the culling of about 2000 hamsters across Hong Kong. The SARS-CoV-2 genome sequence from affected humans and Syrian hamsters belonged to the delta variant (AY.127), and analyses pointed to multiple hamster-to-human transmission events. Interestingly, other animals from the pet shop such as dwarf hamsters, rabbits, guinea pigs, chinchillas and mice tested negative for SARS-CoV-2. This incident is the only documented evidence of Syrian hamsters acquiring SARS-CoV-2 infection in natural settings.93
About 40% of the serum samples from white-tailed deer collected by the United States Department of Agriculture between January 2020 and March 2021 were positive for SARS-CoV-2 antibodies.94 Based on the initial finding, further study was initiated to understand the SARS-CoV-2 strains circulating in deer. Retropharyngeal lymph nodes were collected from white-tailed deer from Iowa between April 2020 and January 2021. Whole-genome sequence of SARS-CoV-2 from white-tailed deer indicated the presence of 12 lineages of SARS-CoV-2, with the predominance of B.1.2 (54.5%), B.1.311 (20%), B.1 (7%) and B.1.234 (6%) lineages. Surprisingly, the B.1.2 lineage was also the most abundant lineage circulating in humans in Iowa during that period, suggesting multiple human-to-deer and deer-to deer transmissions; however, deer-to-human transmission could not be interpreted from this study.95
Brazil has reported the presence of SARS-CoV-2 nucleic acid in wild animals such as Giant anteaters, black-tailed marmoset and West Indian manatee.96 Since these reports were based on random surveillance, the source of infection could not be determined. However, these findings have further widened the list of animals susceptible to SARS-CoV-2 infection.
6 The Veterinary and One Health Perspective
SARS-CoV-2 infection has been confirmed so far in canids (dogs and raccoon dogs), felids (cats, tigers, lions, puma/cougar, lynx and snow leopard), mustelids (ferrets and minks), viverrids (binturongs), procyonids (coatimundis), hyaenids (hyenas), cervids (white-tailed deer, mule deer), cricetids (Syrian hamster), callitrichids (black-tailed marmoset), myrmecophagids (giant anteater), trichechids (West India manatee), castorids (beaver) and primates (gorilla). With such a wide range of hosts available for the virus, it is possible that the virus can mutate, adapt and be transmitted widely.97 The close similarity of the SARS-CoV-2 genome sequence between humans and pet animals and the higher incidence of SARS-CoV-2 infection in pet animals during the human pandemic strongly suggest that humans can transmit SARS-CoV-2 to closely in-contact pets such as cats and dogs. On the other hand, transmission of SARS-CoV-2 from cats or dogs to humans is yet to be established and needs further investigation. Though there is no documented evidence of transmission of SARS-CoV-2 from animals to humans, except from minks and Syrian hamsters, animal owners and veterinarians must be vigilant while handling animals with respiratory illness to minimize the potential risk of transmission from pets.
One way to mitigate transmission from animals is to vaccinate the animals. In addition, this could also save endangered animals. Since SARS-CoV-2 has broad host range, it may be practically impossible to vaccinate all the animals. However, susceptible pet animals and captive animals that are in close proximity with humans should be vaccinated in order to avoid any animal-to-human transmission of SARS-CoV-2. However, very few vaccines have been tested or are available for use in animals. An aluminium-adjuvanted subunit vaccine based on modified S protein was demonstrated to protect minks from SARS-CoV-2 challenge. Upon challenge, the virus was not detected in any of the organs that were tested in the vaccinated group while high titre of the virus was detected in all the organs of control animals.39 Similarly, black-footed ferrets, which are endangered in North America, could produce neutralizing antibodies when immunized with commercially available purified SARS-CoV-2 Spike protein adsorbed to aluminium oxyhydroxide (alhydrogel® 2%). More than 100 captive animals were then vaccinated in a similar way, and the detailed scientific data are awaited.98
Early this year, Russia announced the development and testing of an inactivated SARS-CoV-2-based vaccine named Carnivac-Cov for animals. As per the available information, it has been tested in dogs, cats, minks and foxes and found to be safe and immunogenic.99 In September 2021, the Finnish Breeders' Association (FIFUR) announced in collaboration with the University of Helsinki the development of Furcovac, a vaccine for minks. However, scientific details of the vaccine are not in the public domain.100 Currently, zoo animals and minks in the USA are vaccinated with an adjuvanted SARS-CoV-2 trimeric spike protein-based subunit vaccine.101 Further scientific data are awaited.
Veterinary professionals can not only contribute to control of animal infectious diseases and play a critical role in contributing to the gross domestic product (GDP) of any nation through improving animal health and farmer’s welfare (to quote Mohandas Gandhi, “the greatness of a nation can be judged by the way its animals are treated”), but also are an important cog in the public health system. Along with other disciplines, the veterinary profession has an equal part in approaching zoonotic diseases holistically, and this includes COVID-19.
Indeed, One Health has its beginnings in veterinary medicine. When the cattle plague rinderpest was decimating bovine populations and disrupting the human food supply, Pope Clement XI is reported to have instructed the papal physician Giovanni Lancisi to provide a solution. This led to Lancisi in 1914, followed by Thomas Bates in England, to recommend culling of affected animals and burying their carcasses and implement restrictions in animal movement.102 The contributions of Rudolf Virchow also cannot be ignored. Son of a butcher by profession, he experimented on the lifecycle of the parasite Trichinella in the pig muscle as well as cysticercosis and tuberculosis in cattle. He stated that “between animal and human medicine, there are no dividing lines, nor should there be,” and coined the word ‘zoonosis’.103 In recent times, veterinary public health specialists James Steele and Calvin Schwabe pioneered the holistic approach to infectious disease medicine. The word One Medicine was coined by Calvin Schwabe in the 1970s. As per this concept, both human and veterinary medicine are considered indifferent and contribute to the development of each other. Later, the concept of “One Medicine” was extended to “One Health” through practical application.104
International organizations such as the World Organization for Animal Health (WOAH), the Food and Agriculture Organization (FAO), the World Health Organization (WHO) and the One Health Commission have come together to promote “One Health” and its implementation at various levels.105 In March 2022, the United Nations Environment Programme (UNEP) joined this One Health alliance. As human, animal, plant and environmental health are intricately intertwined, there is need for concerted efforts for cross-sectoral dialogue and cooperation at all levels in order to promote the well-being of humans and animals. Ultimately, we should move towards Planetary Health. However, the risk of zoonosis or their reporting is not uniform worldwide. Tropical, mostly low-to-middle income countries tend to be at greatest risk of zoonoses, and they need to evolve locally feasible solutions, including developing infrastructure, indigenous low cost diagnostics such as lateral flow assays, strengthening of reporting systems and flow of information, improving care systems in the case of disease outbreaks and implementation of preventive measures, including development of locally deployable vaccines and immunization programs.
References
Al-Tawfiq JA, Memish ZA (2020) Middle east respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus. Semin Respir Crit Care Med 41:568–578
Tyrrell CS, Allen JLY, Gkrania-Klotsas E (2021) Influenza: epidemiology and hospital management. Medicine (Abingdon) 49:797–804
Philippon DAM, Wu P, Cowling BJ, Lau EHY (2020) Avian influenza human infections at the human-animal interface. J Infect Dis 222:528–537
Ohimain EI, Silas-Olu D (2021) The 2013–2016 Ebola virus disease outbreak in West Africa. Curr Opin Pharmacol 60:360–365
Anonymous (1996) The status of world health. The world health report 1996—fighting disease, fostering development. The World Health Organization, Geneva
Paules CI, Eisinger RW, Marston HD, Fauci AS (2017) What recent history has taught us about responding to emerging infectious disease threats. Ann Intern Med 167:805–811
Jones KE et al (2008) Global trends in emerging infectious diseases. Nature 451:990–993
Patel S (2017) Every member of the kingdom Animalia is a potential vector of human pathogens. Microb Pathog 109:1–3
Hoffmann M et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280e278
Li Y, Wang H, Tang X, Ma D, Du C, Wang Y, Pan H, Zou Q, Zeng J, Xu L, Farzan M, Zhong G (2020) Potential host range of multiple SARS-like coronaviruses and an improved ACE2-Fc variant that is potent against both SARS-CoV-2 and SARS-CoV-1. bioRxiv
Liu Y, Hu G, Wang Y, Zhao X, Ji F, Ren W, Gong M, Ju X, Li C, Hong J, Zhu Y, Cai X, Wu J, Lan X, Xie Y, Wang X, Yuan Z, Zhang R, Ding Q (2020) Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. bioRxiv
Damas J et al (2020) Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. bioRxiv
Lam SD, Bordin N, Waman VP, Scholes HM, Ashford P, Sen N, van Dorp L, Rauer C, Dawson NL, Pang CSM, Abbasian M, Sillitoe I, Edwards SJL, Fratenali F, Lees JG, Santini JM, Orengo CA (2020) SARS-CoV-2 spike protein predicted to form stable complexes with host receptor protein orthologues from mammals, but not fish, birds or reptiles. bioRxiv
Praharaj MR, Garg P, Khan RIN, Sharma S, Panigrahi M, Mishra BP, Mishra B, Kumar GS, Gandham RK, Singh RK, Majundar S, Mohapatra T (2020) Prediction analysis of SARS-CoV-2 entry in livestock and wild animals. bioRxiv
Zhai X et al (2020) Comparison of SARS-CoV-2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J Virol. https://doi.org/10.1128/JVI.00831-20
Boni MF, Lemey P, Jiang X, Lam TT-K, Perry B, Castoe TM, Rambaut A, Robertson D (2020) Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. bioRxiv
Lau SKP et al (2020) Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. https://doi.org/10.3201/eid2606.200239
Lu R et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574
Zhou P et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
Liu P et al (2020) Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog 16:e1008421
Wahba L et al (2020) An extensive meta-metagenomic search identifies SARS-CoV-2-homologous sequences in pangolin lung viromes. mSphere. https://doi.org/10.1128/mSphere.00160-20
K. Xiao et al., Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature, (2020).
Zhang T, Wu Q, Zhang Z (2020) Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol 30:1578
Lam TT et al (2020) Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 583:282–285
Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (2020) The proximal origin of SARS-CoV-2. Nat Med 26:450–452
Temmam SKV, Salazar EB, Munier S, Bonomi M, Régnault B, Douangboubpha B, Karami Y, Chretien D, Sanamxay D, Xayaphet V, Paphaphanh P, Lacoste V, Somlor S, Lakeomany K, Phommavanh N, Pérot P, Donati F, Bigot T, Nilges M, Rey F, van der Werf S, Brey P, Eloit M (2021) Coronaviruses with a SARS-CoV-2-like receptor-binding domain allowing ACE2-mediated entry into human cells isolated from bats of Indochinese peninsula. Preprint Server: Research Square (2021)
Leist SR et al (2020) A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183:1070-1085e1012
Bao L et al (2020) The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583:830–833
Dinnon KH 3rd et al (2020) A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586:560–566
Hou YJ et al (2020) SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 370:1464–1468
Winkler ES et al (2020) SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21:1327–1335
Jiang RD et al (2020) Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182:50-58e58
Sun SH et al (2020) A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28:124-133e124
Guo M, Tao W, Flavell RA, Zhu S (2021) Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol 18:269–283
Hassan AO et al (2020) A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182:744-753e744
Israelow B et al (2020) Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med 217:e20201241
Chan JF et al (2020) Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 71:2428–2446
Imai M et al (2020) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA 117:16587–16595
Shuai L et al (2021) Replication, pathogenicity, and transmission of SARS-CoV-2 in minks. Natl Sci Rev 8:nwaa291
Shi J et al (2020) Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368:1016–1020
Y. I. Kim et al., Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 27, 704–709 e702 (2020).
Richard M et al (2020) SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun 11:3496
Freuling CM et al (2020) Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerg Infect Dis 26:2982–2985
Halfmann PJ et al (2020) Transmission of SARS-CoV-2 in domestic cats. N Engl J Med 383:592–594
Bosco-Lauth AM et al (2020) Experimental infection of domestic dogs and cats with SARS-CoV-2: pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci USA 117:26382–26388
Ulrich L et al (2021) Experimental SARS-CoV-2 infection of bank voles. Emerg Infect Dis 27:1193–1195
Ulrich L, Wernike K, Hoffmann D, Mettenleiter TC, Beer M (2020) Experimental infection of cattle with SARS-CoV-2. Emerg Infect Dis 26:2979–2981
Zhao Y, Wang J, Kuang D, Xu J, Yang M, Ma C, Zhao S, Li J, Long H, Ding K, Gao J, Liu J, Wang H, Li H, Yang Y, Yu W, Jing Y, Zheng Y, Wu D, Lu S, Liu H, Peng X (2020) Susceptibility of tree shrew to SARS-CoV-2 infection. bioRxiv 2020
Singh DK et al (2021) Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat Microbiol 6:73–86
Rockx B et al (2020) Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015
Palmer MV et al (2021) Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol. https://doi.org/10.1128/JVI.00083-21
Cool K et al (2021) Infection and transmission of SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. bioRxiv
Gaudreault NN et al (2021) Susceptibility of sheep to experimental co-infection with the ancestral lineage of SARS-CoV-2 and its alpha variant. bioRxiv 2021.2011.2015.468720
ProMed-mail (2020) COVID-19 update (56): China (Hong Kong) animal, dog, final serology positive. ProMed
Sit THC, Brackman CJ, Ip SM, Ta KWS, Law PYT, To EMW, Tu VYT, Sims LD, Tsang DNC, Chu DKW, Perera RAPM, Poon LLM, Peiris M (2020) Infection of dogs with SARS-CoV-2. Nature 586:776–778
Williams D (2020) A pug in North Carolina may be the first dot in US to test positive for coronavirus. CNN News (2020)
ProMed-mail (2020) COVID-19 update (58): Belgium, cat, clinical case, RFI. ProMed No. 7151215, International Society for Infectious Diseases
Anonymous (2020) Confirmation of COVID-19 in two pet cats in New York. Press release (United States of America Department of Agriculture
Anonymous (2020) SARS-CoV-2/COVID-19, United States of America. Information received on 22/04/2020 from Dr. Mark Davidson, Associate Administrator, USDA-APHIS, United States Department of Agriculture, Washington, United States of America. OIE report, The World Organisation for Animal Health (OIE).
ProMed-mail (2020) COVID-19 update (51): China (Hong Kong) pet cat. 2020. ProMed (2020)
Zhang Q, Zhang H, Huang K, Yang Y, Hiu X, Gao J, He X, Li C, Gong W, Zhang Y, Peng C, Gao X, Che H, Zhu Z, Shi Z, Jin M (2020) SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv 2020
Temman S, Barbarino A, Maso D, Behillil S, Enouf V, Huon C, Jaraud A, Chevallier L, Backovic M, Perot P, Verwaerde P, Tiret L, van der Werf S, Eliot M (2020) Absence of SARS-CoV-2 infection in cats and dogs in close contact with a cluster of COVID-19 patients in a veterinary campus. bioRxiv 2020
Ruiz-Arrondo I et al (2021) Detection of SARS-CoV-2 in pets living with COVID-19 owners diagnosed during the COVID-19 lockdown in Spain: a case of an asymptomatic cat with SARS-CoV-2 in Europe. Transbound Emerg Dis 68:973–976
Deng J et al (2020) Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound Emerg Dis 67:1745–1749
Calvet GA et al (2021) Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS One 16:e0250853
Ferasin L et al (2021) Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet Rec 189:e944
Dileepan M et al (2021) Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 12:1597–1609
Fritz M et al (2021) High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health 11:100192
Jara LM et al (2021) Evidence of neutralizing antibodies against SARS-CoV-2 in domestic cats living with owners with a history of COVID-19 in Lima—Peru. One Health 13:100318
Klaus J et al (2021) SARS-CoV-2 infection in dogs and cats from southern Germany and northern Italy during the first wave of the COVID-19 pandemic. Viruses 13:1453
Krafft E et al (2021) Report of one-year prospective surveillance of SARS-CoV-2 in dogs and cats in France with various exposure risks: confirmation of a low prevalence of shedding, detection and complete sequencing of an alpha variant in a cat. Viruses 13:1759
Sailleau C et al (2020) First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound Emerg Dis 67:2324–2328
Medkour H et al (2021) First evidence of human-to-dog transmission of SARS-CoV-2 B1160 variant in France. Transbound Emerg Dis. https://doi.org/10.1111/tbed.14359
Klaus J et al (2021) Detection and genome sequencing of SARS-CoV-2 in a domestic cat with respiratory signs in Switzerland. Viruses 13:496
Fuentealba NA et al (2021) First detection and molecular analysis of SARS-CoV-2 from a naturally infected cat from Argentina. Vet Microbiol 260:109179
Yaglom HD et al (2021) Genomic investigation of a household SARS-CoV-2 disease cluster in Arizona involving a cat, dog, and pet owner. One Health 13:100333
Schulz C et al (2021) SARS-CoV-2-specific antibodies in domestic cats during first COVID-19 wave, Europe. Emerg Infect Dis 27:3115–3118
OIE (2021) COVID-19 events in animals. World Organization for Animal Health
Bartlett SL et al (2021) Sars-Cov-2 Infection and Longitudinal Fecal Screening in Malayan Tigers (Panthera Tigris Jacksoni), Amur Tigers (Panthera Tigris Altaica ), and African Lions (Panthera Leo Krugeri) at the Bronx Zoo, New York, USA. J Zoo Wildl Med 51:733–744
Wang L et al (2020) Complete genome sequence of SARS-CoV-2 in a tiger from a U.S. zoological collection. Microbiol Resour Announc 9:e00468-20
USDA (2021) Confirmed cases of SARS-CoV-2 in animals in the United States
ProMed-mail (2021) Coronavirus disease 2019 update (348): animal, USA, zoo, snow leopard, fatal, suspected 13 October 2021. International Society for Infectious Diseases.
ProMed-mail (2021) Coronavirus disease 2019 update (392): animal, USA, zoo, snow leopard, fatal 15 November 2021. International Society for Infectious Diseases
Molenaar RJ et al (2020) Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Vet Pathol 57:653–657
Oreshkova N et al (2020) SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 25:2001005
ProMed-mail (2020) COVID-19 update (189): Netherlands (North Brabant) Animal, Farmed Mink, Research, Cat, Dog. 2020. ProMed
van Aart AE et al (2021) SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound Emerg Dis. https://doi.org/10.1111/tbed.14173. Online ahead of print. Jun 3
Hammer AS et al (2021) SARS-CoV-2 Transmission between Mink (Neovison vison) and Humans, Denmark. Emerg Infect Dis 27:547–551
CDC (2021) Animals and COVID-19
Devaux CA et al (2021) Spread of mink SARS-CoV-2 variants in humans: a model of sarbecovirus interspecies evolution. Front Microbiol 12:675528
Mallapaty S (2020) COVID mink analysis shows mutations are not dangerous—yet. Nature 587:340–341
OIE (2020) SARS-CoV-2 infection in mink in northern Greece. OIE—World Organisation for Animal Health
Yen HL et al (2022) Transmission of SARS-CoV-2 delta variant (A.Y127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet 399:1070–1078
Chandler JC et al (2021) SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci USA 118:e2114828118
Kuchipudi SV et al (2021) Multiple spillovers and onward transmission of SARS-Cov-2 in free-living and captive white-tailed deer (Odocoileus virginianus). bioRxiv, 2021.2010.2031.466677
WOAH/OIE (2022) SARS-CoV-2 in animals (Inf. with), Brazil. World Organisation for Animal Health
Banerjee A, Mossman K, Baker ML (2021) Zooanthroponotic potential of SARS-CoV-2 and implications of reintroduction into human populations. Cell Host Microbe 29:160–164
AAHA, "Endangered black-footed ferret gets experimental COVID-19 vaccine in Colorado," (American Animal Hospital Association, 2020).
WSAVA, "COVID-19 - An update for WSAVA Members April 29th, 2021," (World Small Animal Veterinary Association, Ontario, Canada, 2021).
FIFUR (2021) FIFUR has obtained a conditional usage permit for mink vaccine from the Finnish Food Authority. Finnish Fur Breeders' Association, Finland
Sharun K, Tiwari R, Saied ARA, Dhama K (2021) SARSCoV- 2 vaccine for domestic and captive animals: an effort to counter COVID-19 pandemic at the human-animal interface. Vaccine 39:7119–7122
Diallo A (1988) 'Rinderpest' and 'peste des petits ruminants': constant threats to animal farming in many developing countries. Impact of Science on Society, UNESCO
Schultz M (2008) Rudolf Virchow. Emerg Infect Dis 14:1480–1481
Zinsstag J, Schelling E, Waltner-Toews D, Tanner M (2011) From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med 101:148–156
FAO (2019) Taking a multisectoral one health approach : a tripartite guide to addressing zoonotic diseases in countries. FAO/OIE/WHO
Munster VJ et al (2020) Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272
Deng W et al (2020) Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 369:818–823
Chandrashekar A et al (2020) SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369:812–817
Zhang Q et al (2020) A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg Microbes Infect 9:2013–2019
McAloose D et al (2020) From people to panthera: natural SARS-CoV-2 infection in tigers and lions at the bronx zoo. MBio. https://doi.org/10.1128/mBio.02220-20
Oude Munnink BB et al (2021) Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371:172–177
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial or other conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, D., Bayry, J. & Hegde, N.R. COVID-19: A Veterinary and One Health Perspective. J Indian Inst Sci 102, 689–709 (2022). https://doi.org/10.1007/s41745-022-00318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-022-00318-9