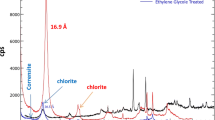
Abstract
The modified clay was used for organic compounds adsorption and removal. However, desorption of the organic molecules, used for clay modification, in the real internal digestion environment of the animals is the main concern. Therefore, in this study, the adsorption/desorption studies of tri-capryl methyl ammonium chloride (TCMA)-modified clay at different cation exchange capacity (CEC) were investigated either by washing or by exposure to electrolytic solutions. The results indicated that the TCMA adsorption reaches up to 3 times the CEC of this clay. Beyond the CEC, the adsorption is accomplished by hydrophobic interaction. It is also found that the washing of clay has no effect on desorption of TCMA. Furthermore, desorption studies with stomach electrolyte solutions showed no more than 2 mg/L desorption of TCMA at acidic and neutral pH values which indicates the suitability of the TCMA clay for mycotoxin removal.
Article Highlights
-
Organophilic clay was used for removal of organic molecules and mycotoxins.
-
Adsorption/desorption of TCMA was tested.
-
Adsorption of TCMA is a CEC dependent.
-
The washing of clay has no effect on desorption of TCMA.
-
With stomach electrolytes, < 2 mg/L desorption of TCMA at acidic, and neutral pH values.










Similar content being viewed by others
References
Abbès S, Ouanes Z, Salah-Abbès JB, Houas Z, Oueslati R, Bacha H et al (2006) The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zearalenone in mice. Toxicon 47(5):567
Afriyie-Gyawu E, Wiles MC, Huebner HJ, Richardson MR, Fickey C, Phillips TD (2005) Prevention of zearalenone-induced hyperestrogenism in prepubertal mice. J Toxicol Environ Health Part A Curr Issues 68(5):353–368
Avantaggiato G, Havenaar R, Visconti A (2003) Assessing the zearalenone-binding activity of adsorbent materials during passage through a dynamic in vitro gastrointestinal model. Food Chem Toxicol 41(10):1283
Cavalcanti JL, Abreu CM, Baraúna OS, da Motta M (2014) Phenol adsorption by modified clay from Northeastern Brazil. Can J Chem Eng 92:355–361
Chen G, Song X, Zhao Y, Han B, Yan H (1997) Adsorption enthalpy and adsorption isotherm of tetradecylpyridinium bromide on Na-montmorillonite. J Colloid Interface Sci 186(1):206
Dakovic A, Tomasevic-Canovic M, Dondur V, Rottinghaus GE, Medakovic V, Zaric S (2005) Adsorption of mycotoxins by organozeolites. Colloids Surf B 46(1):20
Dakovic A, Matijasevic S, Rottinghaus GE, Dondur V, Pietrass T, Clewett CFM (2007) Adsorption of zearalenone by organomodified natural zeolitic tuff. J Colloid Interface Sci 311(1):8–13
de Paiva LB, Morales AR, Valenzuela Díaz FR (2008) Organoclays: properties, preparation and applications. Appl Clay Sci 42(1–2):8
Dixon JB, Kannewischer I, Tenorio Arvide MG, Barrientos Velazquez AL (2008) Aflatoxin sequestration in animal feeds by quality-labeled smectite clays: an introductory plan. Appl Clay Sci 40(1–4):201
(2006) Handbook of clay science. In: Faïza Bergaya BKG, Lagaly TG (Eds), 1st edn. vol.1, Developments in Clay Science Series, Elsevier Science, New York
Hang PT, Brindley GW (1970) Methylene blue absorption by clay minerals. Determination of surface areas and cation exchange capacities (clay-organic studies xviii). Clays Clay Miner 18:203–212
Ismadji S, Soetaredjo FE, Ayucitra A (2015) Clay materials for environmental remediation, chapter 3: modification of clay minerals for adsorption purpose. Springer, New York
Karaca S, Gürses A, Açışlı Ö, Hassani A, Kıranşan M, Yıkılmaz K (2013) Modeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clay. Desalin Water Treat 51:2726–2739
Kong C, Shin SY, Kim BG (2014) Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: an in vitro approach. SpringerPlus 3:346–351
Kwolek T, Hodorowicz M, Stadnicka K, Czapkiewicz J (2003) Adsorption isotherms of homologous alkyldimethylbenzylammoniumbromides on sodium montmorillonite. J Colloid Interface Sci 264(1):14
Lagaly G (1986) Interaction of alkylamines with different types of layered compounds. Solid State Ion 22(1):43–51
Lemke SL, Mayura K, Reeves WR, Wang N, Fickey C, Phillips TD (2001) Investigation of organophilic montmorillonite clay inclusion in zearalenone-contaminated diets using the mouse uterine weight bioassay. J Toxicol Environ Health Part A 62(4):243–258
Neeff DV, Ledoux DR, Rottinghaus GE, Bermudez AJ, Dakovic A, Murarolli RA, Oliveira CAF (2013) In vitro and in vivo efficacy of a hydrated sodium calcium aluminosilicate to bind and reduce aflatoxin residues in tissues of broiler chicks fed aflatoxin B1. Poult Sci 92:131–137
Pappas AC, Tsiplakou E, Georgiadou M, Anagnostopoulos C, Markoglou AN, Liapis K, Zervas G (2014) Bentonite binders in the presence of mycotoxins: results of in vitro preliminary tests and an in vivo broiler trial. Appl Clay Sci 99:48–53
Patzko A, Dekany I (1993) Ion-exchange and molecular adsorption of a cationic surfactant on clay-minerals. Colloids Surf A Physicochem Eng Asp 71(3):299–307
Praus P, Turicova M (2007) A physico-chemical study of the cationic surfactants adsorption on montmorillonite. J Braz Chem Soc 18(2):378–383
Rytwo G, Serban C, Nir S, Margulies L (1991) Use of methylene-blue and crystal violet for determination of exchangeable cations in montmorillonite. Clays Clay Miner 39(5):551–555
Tahani A, Karroua M, Van Damme H, Levitz P, Bergaya F (1999) Adsorption of a cationic surfactant on Na-montmorillonite: inspection of adsorption layer by X-ray and fluorescence spectroscopies. J Colloid Interface Sci 216(2):242–249
Wang G, Miao Y, Sun Z, Zheng S (2018) Simultaneous adsorption of aflatoxin B-1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J Colloid Interface Sci 511:67–76
Zhang ZZ, Sparks DL, Scrivner NC (1993) Sorption and desorption of quaternary amine cations on clays. Environ Sci Technol 27(8):1625–1631
Acknowledgements
The authors are gratefully thanks Particle Engineering Research Center (PERC), University of Florida, Gainesville, Florida, USA for supproting this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Hue, KA.A., El-Midany, A.A. & El-Shall, H.E. Adsorption/Desorption Stability of TCMA-Modified Clay in Simulated Digestion Environment. Int J Environ Res 13, 879–885 (2019). https://doi.org/10.1007/s41742-019-00227-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00227-3