Abstract
Introduction
Incidence and mortality for bladder cancer has changed very little over the past 20 years. Approximately 40% of patients with high-risk nonmuscle invasive bladder cancer eventually recur/progress. It is important to understand the economic impact of disease recurrence/progression in bladder cancer. Our aim was to estimate and understand the direct costs associated with the treatment of bladder cancer from the payer’s perspective in the USA, in the year of 2021, including costs for both newly diagnosed bladder cancer (stages 0a–IV) and recurrent patients.
Methods
An economic model was constructed to calculate the number of patients receiving each treatment modality at every stage of disease and their respective costs. Epidemiological data were based on the CancerMPact Patient Metrics (PM) database and treatment modality data retrieved from CMP Treatment Architecture (TA), 2021 version. Resource utilization and costs were obtained from medical literature and public data sources. Only direct costs were considered.
Results
There were an estimated 83,532 newly diagnosed patients with bladder cancer of all stages in 2021 with a projected total cost of treatment of ~$2.6 billion. Average cost per newly diagnosed patient varied from $19,521 (stage 0a) to $169,533 (metastatic disease). Cost profile differed substantially among the stages of disease. For the 75,760 patients that were expected to have a recurrence in 2021, an additional cost of ~$3.9 billion was estimated at an average cost per patient of $52,179. The expected total cost to treat newly diagnosed and newly recurrent patients is reported in this model, with the total cost in 2021 estimated to exceed $6.5 billion.
Conclusions
Treatment and resource costs increase for bladder cancer as the disease recurs/progresses. More effective treatments that can delay recurrence/progression may reduce the economic burden associated with bladder cancer.
Plain Language Summary
The costs of treating bladder cancer in the USA are high, expected to have exceeded $6.5 billion in the year 2021. The average cost for newly diagnosed patients ranges from $19,521 for the earliest stage of disease to $169,533 for metastatic disease. Average costs per patient increase upon disease recurrence and more effective initial treatments to delay disease recurrence or progression may reduce the costs associated with bladder cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bladder cancer is the sixth most common cancer in the USA with an incidence of around 83,000 patients and 17,000 deaths per year [1, 2]. Predominant risk factors include male gender, advanced age, and history of tobacco use [2, 3]. Bladder cancer is a complex and financially burdensome disease, ranking among the top ten most expensive cancers to treat over a patient’s lifetime in the USA [4]; it is estimated that more than 4 billion US dollars are spent yearly in the diagnosis, surveillance, and treatment of this disease [5, 6].
Around 90% of bladder cancers are urothelial carcinomas, which originate in the urinary tract’s epithelial lining. Lesions confined to the bladder’s innermost tissue that have not invaded the muscularis layer of the bladder are classified as nonmuscle invasive bladder cancer (NMIBC) while lesions that have penetrated the surrounding muscle layer are defined as muscle invasive bladder cancer (MIBC) [2, 7, 8].
NMIBCs are early-stage carcinomas (Stages 0a–I) that account for approximately 75% of newly diagnosed bladder cancer [2, 7,8,9]. These patients typically undergo transurethral resection of bladder tumors (TURBT) for diagnosis, staging, and grading, with tumor grade being a crucial prognostic indicator. NMIBC, known for high recurrence rates, sees variable progression to muscle-invasive bladder cancer (MIBC) based on initial tumor grade and classification. Low-grade Ta lesions have a 30–60% 5-year recurrence rate, with a ≤ 5% MIBC progression risk [2, 7,8,9]. High-grade T1 lesions face 30–70% progression rates, with over 80% recurrence and 50% advancing to MIBC within three years [2, 7,8,9]. Patients with NMIBC are stratified into low, intermediate, and high-risk categories, which determine their management strategies, from active surveillance in low-risk cases to BCG induction and ongoing maintenance therapy for those at higher risk. This stratification informs and tailors the therapeutic regimen to the patient’s specific risk level [2, 7,8,9].
MIBC (stages II–IVA) presents a more aggressive course, treated with cystectomy, radiotherapy, and systemic therapy, aiming for long-term remission. Stage IVb, or metastatic bladder cancer, is managed with systemic chemotherapy and targeted therapies, prioritizing palliation and quality of life due to generally poor prognoses. Overall, bladder cancer has a 77% survival rate, with a > 90% chance of long-term remission for early-stage, low-grade tumors but only an 8% 5-year survival for advanced metastatic disease [2, 7,8,9].
The complex and prolonged clinical approach necessary to treat bladder cancer, along with the high incidence of this disease in the US, results in significant economic burden for patients as well as the medical system [10]. Bladder cancer is a complex and costly cancer with a high lifetime cost [5, 6] . Several cost studies focusing on bladder cancer in the USA have been published, but very few have evaluated the total cost of the disease to the USA healthcare system, from stages 0 to IVB and relapsed bladder cancer. In general, there is a paucity of data regarding the costs to treat bladder cancer in the country [5, 6].
The objective of this study is to estimate and elucidate the direct costs related to the treatment of bladder cancer across all stages (0a–IVB) including relapsed cases, from the perspective of US payers for the year 2021. Our analysis encompasses both patients newly diagnosed with bladder cancer and those anticipated to experience recurrence or relapse in the same year.
2 Methods
2.1 Data Sources
CancerMPact (CMP) is a proprietary database from Oracle that includes a dataset of Epidemiological data [patient metrics (PM)] and data about the patterns of care and outcomes of tumors obtained from a physician survey [treatment architectures (TA)] [1, 11]:
-
Treatment patterns, regimen preferences, and clinical outcomes were obtained from the CMP TA Bladder Cancer US survey, edition 2021 [7]. This survey creates a comprehensive treatment pathway that maps patient flow, providing detailed insights into disease outcomes and progression at each treatment stage. This pathway delineates the proportion of patients at a particular stage undergoing various treatments, including surgery, BCG, surveillance, chemotherapy, among others, and the outcomes they experience, such as responses, relapses, and progression. The information spans across several treatment lines, including relapsed/recurrent patients and enables a nuanced understanding of treatment dynamics. In 2021, 90 physicians from the USA were surveyed about bladder cancer. The sample was composed of 40% urologists and 60% oncologists who were in clinical practice for an average of 17 years and from all regions of the country. Physicians reported treating an average of 37 patients with bladder cancer per month.
-
Incidence and recurrence rates of bladder cancer for 2021 in the USA were extracted from the CMP PM database [1]. PM encompasses a comprehensive database that houses detailed epidemiology, staging, and treatment information, along with projections, for up to 33 types of tumors across the USA, Western Europe (including the United Kingdom, Italy, France, Germany, and Spain), Japan, and China. Utilizing local cancer registry databases within each country, PM estimates critical data, such as the incidence and prevalence of these tumors, broken down by stage. For this project, incidence and prevalence data were extracted for the year 2021 within the USA.
-
Resource utilization and costs were calculated based on published data and literature [12,13,14,15,16,17]. The department of Veterans Affairs (VA) database was the preferential source of costs, as it is publicly available and has a comprehensive list of resources and associated costs [18]. We also used data from the Medicare Physician Fee Schedule to calculate medical fees [19].
2.2 Decision Tree Model
A static decision tree model was built using Microsoft Excel to calculate the total number of patients that received a given treatment and the costs associated with each of the treatment strategies. The model includes newly diagnosed patients with bladder cancer from stage 0a to IVB and those that were expected to relapse/recur in 2021. At the model entry, the estimated number of newly-diagnosed patients for 2021 were distributed according to their cancer staging, with data obtained from CMP PM (Table 1) [1]. A parallel process was implemented for recurrent or relapsed patients, integrating their numbers from CMP PM with detailed treatment strategies from CMP TA [1, 7]. The model calculates, for each bladder cancer stage, the distribution of patients across various treatment options based on real-world usage data from CMP TA [7].
We subsequently determined the costs associated with each specific treatment and procedure outlined in our decision tree. By aligning these costs with the respective patient numbers, we were able to compute the aggregate treatment costs for each stage and branch within the decision tree, ensuring a comprehensive cost analysis across the different pathways. Only direct costs of health procedures arising from bladder cancer and its complications were considered in the model, including, but not limited to, laboratory and imaging tests, ambulatory visits, cystectomy, TURBT, radiotherapy, systemic chemotherapy, and treatment-related adverse events. All costs were input and calculated in USD as reported by the original sources. We utilized the 2021 base prices for drugs and medical procedures as sourced from the Veterans Affairs (VA) and Medicare networks (Supplementary Material). Additionally, we incorporated data from studies published in 2020 and 2021 (Supplementary Table 2). We did not adjust these prices given their recency. For both the incidence and recurrence models, total costs for treatments received in 2021 were calculated. In case of metastatic disease, we did consider up to three lines of therapy, taking into account line of therapy progression numbers from CMP TA [7].
To manage the model’s complexity while maintaining its ability to represent disease progression, and acknowledging that infrequent events minimally affect outcomes, we established a 5% threshold for adverse events [20]. This cutoff was used to determine the inclusion of adverse events parameters in the model.
3 Results
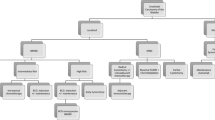
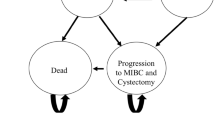
In 2021, the USA witnessed an estimated 83,532 new diagnoses of bladder cancer, accompanied by an additional 75,760 cases of bladder cancer recurrence or progression [1]. The treatment pathway for bladder cancer is highly complex, and patients were treated with a wide range of strategies and received multiple treatments over the course of the disease as captured in the Sankey diagram (Fig. 1) and model schematic (Fig. 2).
Sankey diagram of real-world treatment patterns in patients with bladder cancer. TURBT, transurethral resection of a bladder tumor; RT, radiotherapy; full course, patients that received TURBT followed by BCG intravesical injections for 6–8 weeks; single dose, patients that received a single dose of BCG intravesical
Model structure for bladder cancer based on the patterns of care for the USA, using CancerMPact data for 2021. Cis, cisplatin; TURBT, transurethral resection of a bladder tumor; full course, patients that received BCG intravesical injections for 6–8 weeks; single dose, patients that received a single dose of BCG intravesical
Of newly diagnosed patients, 75% (62,784) presented with NMIBC (stages 0a–I); 20% (16,820) with MIBC (stages II–IVA), and 5% (3928) with metastatic disease (Table 1). Our model estimates that the total cost to treat these patients was ~$2.6 billion in 2021 with an average cost of $30,944 per patient. The average cost varied substantially across different stages of the disease: patients with NMIBC incurred lower costs, ranging from $19,521 to $23,577 per patient. In contrast, the cost for patients with MIBC was estimated between $35,545 and $37,204 per patient, and those with metastatic disease faced the highest treatment costs, averaging $169,553 per patient. This differential cost distribution across stages is further elaborated in Fig. 3 and Table 1.
Cost composition of medical care for patients with bladder cancer in the USA in 2021, by stage. Values represent the cost share for medical care (treatment, adverse events, diagnosis) within a given stage. It is defined as the unit cost for a given type of medical care, weighted by the volume of patients undergoing such treatment
For patients with NMIBC, over half of the total cost was attributed to diagnostic and surveillance activities, highlighting a substantial proportion of patients undergoing surveillance only (37.7% for stage 0a, 14.9% for stage 0 is, 14.9% for stage I) with relatively lower expenses associated with intravesical BCG or chemotherapy treatments. For patients with MIBC, treatment costs corresponded to more than 60% of total costs, and for metastatic patients this percentage was even higher (92.3%). The costs for treatment (excluding diagnosis and surveillance) also differed among the stages (Table 1). While for NMIBC drug costs and surgery each contributed almost equally to total treatment costs, for MIBC drug costs accounted for 26–38%, surgery for 31–48%, and radiotherapy for 17–21% of treatment costs. In contrast, for patients with metastatic disease, drug costs accounted for 75% of total treatment costs. Costs related to adverse events did not exceed 10% of total treatment costs for any stage of disease.
In 2021, bladder cancer recurrences were anticipated in 75,760 patients, with 62% experiencing NMIBC, 28% MIBC, and 10% metastatic recurrences (Table 1). The cost distribution for treating recurrent bladder cancer reveals a higher allocation toward treatment procedures over diagnosis and surveillance across all disease stages. Specifically, drug costs contributed to 29.6%, 46.1%, and 74.6% of the total treatment costs for NMIBC, MIBC, and metastatic recurrences, respectively. Surgical interventions accounted for a significant portion of costs in NMIBC and MIBC recurrences, 30.1% and 27.1% respectively. Consequently, the financial burden for treating recurrent bladder cancer in this cohort approached approximately $3.9 billion.
4 Discussion
Our analysis estimated that more than $6.5 billion were spent in 2021 to treat new and recurrent patients with bladder cancer in the USA, what is similar to a study published by Mariotto et al. [4] in 2019, that estimated the bladder cancer cost in the USA to be $7.54 billion. The model showed that the cost profile varied based on the stage of disease. Average costs for patients with metastatic disease were much higher than costs for those patients with NMIBC or MIBC. This was true for both newly diagnosed patients as well as those patients presenting with a recurrence or progression. These findings are in accordance with a previous study that evaluated the costs of bladder cancer care to Medicare [21].
The contributing factors to total costs also differed according to the stages: costs for NMIBC were mostly associated with diagnostic and surveillance procedures while for patients with metastatic disease major drivers of cost were treatment related. Patients with MIBC were positioned in between, with surgical procedures being a higher driver of costs in this population. The high impact of surveillance procedures and local treatment in the costs of NMIBC has been recognized for more than 20 years [6, 16, 22, 23] and, as our data confirmed, has not changed. Several studies have evaluated the cost-effectiveness of various treatment strategies to help guide clinicians and payers in the market [5, 15, 24, 25]. For example, intravesical maintenance therapy with BCG is a mainstay of NMIBC treatment, and recent evidence suggests that BCG maintenance should be prioritized for patients with high-risk disease, as they are more likely to progress [15]. The global BCG shortage that started in 2019 due to a reduction in pharmaceutical manufacturers has inadvertently led to this prioritization of high-risk patients for BCG therapy when shortages exist, as well as reductions in maintenance BCG [26, 27].
This model showed a higher average cost of treatment for patients with metastatic bladder cancer than that reported in some previous publications: while we found that the average cost to the system for stage IVB patients was almost $170,000, other publications have found this cost to be lower. Aly et al. [10] and Yeung et al. [5] found an average lifetime cost for patients with stage IV bladder cancer to be closer to $120,000 and $107,000 dollars, respectively, while Sloan et al. [21] estimated the cost to be close to $50,000 dollars for the first year of treatment. Possible explanations for these differences are the timing of the studies and the methods used, including differences in payers. All three studies [5, 10, 21] were performed before the use of immunooncology became standard of care in bladder cancer.
The financial impact of bladder cancer management is substantial and varies significantly by region, with the USA experiencing notably higher costs than other countries [28]. A pan-European analysis by Leal et al. highlighted that bladder cancer represents 5% of total healthcare cancer expenditures across Europe, with an average cost per patient of €6942 (equivalent to $7526 in May 2024) [28]. France reported the highest national average cost at €11,937 ($12,904) per patient [28], while Quignot’s research in Germany documented the annual costs of treating NMIBC at €9250 ($10,028) and MIBC at €17,893 ($19,400) [29]. Similarly, Cox’s study in the UK calculated the cost of NMIBC treatment at approximately £8735 ($11,750) per patient [30]. Our model’s projections for the USA substantially surpass these figures (Table 1), confirming a pronounced disparity in bladder cancer management costs between the USA and other countries.
The analysis of patients with recurrent bladder cancer, highlights that recurrence drives up the costs of bladder cancer to the healthcare system. Total costs to treat recurrence are more than 50% higher than those to treat primary disease in our model. Mossanen et al. [16] had called attention to this fact in 2019, when their study showed that progressive disease contributed to 81% and 92% of the overall 5 years cost for intermediate- and high-risk NMIBC. This increase in cost for recurrent disease is driven both by the increased likelihood of disease recurrence with higher stage at initial diagnosis and the increased likelihood of a recurrence being at a higher stage than initial diagnosis [2, 14, 16]. Additionally, patients with recurrent disease are treated more aggressively, further increasing costs [2, 14, 16].
Given the high rates of recurrence seen in clinical practice (51–66%) [7] and the higher cost of treating the disease as it progresses and/or recurs described here and elsewhere [14, 16], an unmet need exists for treatments that could create a durable response with higher rates of complete response, reducing the likelihood of or delaying progression/recurrence. Some promising new diagnostics and therapies being investigated include urinary biomarkers, genomic profiling, and new intravesical and immunotherapy combination protocols which have the potential to improve the care and outcomes of patients with bladder cancer [16, 31,32,33]. While new therapies for NMIBC and MIBC are likely to increase costs for non-metastatic bladder cancer, they may be cost effective if they could prevent or delay progression to metastatic disease for which outcomes are poor [2, 3].
This study has a number of limitations. First, the model included patients diagnosed in 2021 as well as those expected to experience a recurrence/relapse within the same year. However, it does not account for patients who were under surveillance without confirmed evidence of disease. Surveillance strategies are known to be costly and can significantly impact the overall cost of managing patients with bladder cancer [6]. This consideration is crucial for the interpretation of our findings and must be integrated into any examination of our data, given that it may lead to an underestimation of our overall cost projections. Most of the information used to build the clinical evolution, patterns of care, and outcomes of the model, were collected from a physician survey. While this approach provides direct insights from healthcare providers, it is inherently limited by the potential for recall bias [34]. This bias may affect the accuracy of the reported practices and outcomes, introducing a layer of uncertainty to our findings. Another critical limitation stems from our reliance on the Department of Veterans Affairs (VA) database [18] as the primary source of cost data. While the VA database offers a robust and publicly accessible resource and is largely used in economic analyses in the USA, it may not accurately reflect the broader national cost landscape. The VA’s unique pricing negotiations and federal subsidization afford it lower costs for services and medications, which may not be representative of costs encountered in private or other public healthcare systems across the USA. This discrepancy can lead to an underestimation of the actual costs associated with bladder cancer management on a national scale, as costs in other healthcare settings can vary widely based on regional, insurance, and provider network differences [35]. Despite our team conducting a thorough internal validation analysis, involving expert consultations and benchmarking our findings against existing literature, we acknowledge that the absence of external model validation represents a limitation of our study. And finally, the absence of sensitivity analysis in our economic model limits the robustness of our conclusions, as it prevents the assessment of how variations in key assumptions may impact the results [36].
5 Conclusions
This study confirms that costs for bladder cancer in 2021 were high and treatment and resource costs increase as the disease recurs and progresses. More effective treatments and diagnostic strategies in early-stage disease that can prevent and delay recurrence and progression may reduce the economic burden associated with bladder cancer. Future studies examining how improved early treatment options may offset lifetime bladder cancer costs through improved efficacy are needed.
References
Cerner Enviza. CMP: CancerMPact®, Patient Metrics® 2022. www.cancermpact.com. Accessed 23 Apr 2022.
National Cancer Institute—NCI. Bladder Cancer Treatment (PDQ®)–Health Professional Version 2022. https://www.cancer.gov/types/bladder/hp/bladder-treatment-pdq. Accessed 23 Apr 2022.
Farling KB. Bladder cancer: risk factors, diagnosis, and management. Nurse Pract. 2017;42(3):26–33.
Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomark Prev. 2020;29(7):1304–12.
Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32(11):1093–104.
Joyce DD, Sharma V, Williams SB. Cost-effectiveness and economic impact of bladder cancer management: an updated review of the literature. Pharmacoeconomics. 2023;41(7):751–69.
Cerner Enviza. CMP: CancerMPact®, Treatment Architecture Bladder Cancer 2021. www.cancermpact.com. Accessed 23 Apr 2022.
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198(3):552–9.
Garg T, McMullen CK, Leo MC, O’Keeffe-Rosetti MC, Weinmann S, Nielsen ME. Predicting risk of multiple levels of recurrence and progression after initial diagnosis of nonmuscle-invasive bladder cancer in a multisite, community-based cohort. Cancer. 2021;127(4):520–7.
Aly A, Johnson C, Doleh Y, Chirikov V, Botteman M, Shenolikar R, et al. The real-world lifetime economic burden of urothelial carcinoma by stage at diagnosis. J Clin Pathw. 2020;6(4):51–60.
Cerner Enviza. CMP: CancerMPact®, Treatment Architecture® 2022. www.cancermpact.com. Accessed 23 Apr 2022.
Wymer KM, Sharma V, Saigal CS, Chamie K, Litwin MS, Packiam VT, et al. Cost-effectiveness analysis of pembrolizumab for bacillus calmette-guérin-unresponsive carcinoma in situ of the bladder. J Urol. 2021;205(5):1326–35.
Williams SB, Shan Y, Jazzar U, Mehta HB, Baillargeon JG, Huo J, et al. Comparing survival outcomes and costs associated with radical cystectomy and trimodal therapy for older adults with muscle-invasive bladder cancer. JAMA Surg. 2018;153(10):881–9.
Williams SB, Howard LE, Foster ML, Klaassen Z, Sieluk J, De Hoedt AM, et al. Estimated costs and long-term outcomes of patients with high-risk non-muscle-invasive bladder cancer treated with bacillus calmette-guérin in the veterans affairs health system. JAMA Netw Open. 2021;4(3): e213800.
Sharma V, Wymer KM, Borah BJ, Saigal CS, Litwin MS, Packiam VT, et al. Cost-effectiveness of maintenance bacillus calmette-guérin for intermediate and high risk nonmuscle invasive bladder cancer. J Urol. 2020;204(3):442–9.
Mossanen M, Wang Y, Szymaniak J, Tan WS, Huynh MJ, Preston MA, et al. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37(10):2059–65.
Malangone-Monaco E, Wilson K, Diakun D, Tayama D, Satram S, Ogale S. Cost of cystectomy-related complications in patients with bladder cancer in the United States. Curr Med Res Opin. 2020;36(7):1177–85.
U.S. Department of Veterans Affairs (VA). VA Cost Data Overview: US Department of Veterans Affairs; 2023. https://www.herc.research.va.gov/include/page.asp?id=data-overview. Accessed 4 Apr 2023.
Centers for Medicare & Medicaid Services. Physician Fee Schedule: CMS.GOV; 2021. https://www.cms.gov/medicare/payment/fee-schedules/physician. Accessed 1 Feb 2022.
Subramanian J, Simon R. Overfitting in prediction models—is it a problem only in high dimensions? Contemp Clin Trials. 2013;36(2):636–41.
Sloan FA, Yashkin AP, Akushevich I, Inman BA. The cost to medicare of bladder cancer care. Eur Urol Oncol. 2020;3(4):515–22.
Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68(3):549–53.
Heijnsdijk EAM, Nieboer D, Garg T, Lansdorp-Vogelaar I, de Koning HJ, Nielsen ME. Cost-effectiveness of surveillance schedules in older adults with non-muscle-invasive bladder cancer. BJU Int. 2019;123(2):307–12.
Qin S, Yi L, Li S, Tan C, Zeng X, Wang L, et al. Cost-effectiveness of atezolizumab plus chemotherapy as first-line therapy for metastatic urothelial cancer. Adv Ther. 2021;38(6):3399–408.
Sarfaty M, Hall PS, Chan KKW, Virik K, Leshno M, Gordon N, et al. Cost-effectiveness of pembrolizumab in second-line advanced bladder cancer. Eur Urol. 2018;74(1):57–62.
Harvey M, Chislett B, Perera M, Lawrentschuk N, Bolton D, Jack G. Critical shortage in BCG immunotherapy: how did we get here and where will it take us? Urol Oncol. 2022;40(1):1–3.
Balasubramanian A, Gunjur A, Weickhardt A, Papa N, Bolton D, Lawrentschuk N, et al. Adjuvant therapies for non-muscle-invasive bladder cancer: advances during BCG shortage. World J Urol. 2022;40(5):1111–24.
Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder cancer across the European Union. Eur Urol. 2016;69(3):438–47.
Quignot N, Jiang H, Doobaree IU, Lehmann J, Ghatnekar O. Healthcare resource utilization and cost burden of bcg-treated non-muscle invasive bladder cancer patients in Germany: a retrospective claims analysis. Clinicoecon Outcomes Res. 2023;15:227–37.
Cox E, Saramago P, Kelly J, Porta N, Hall E, Tan WS, et al. Effects of bladder cancer on uk healthcare costs and patient health-related quality of life: evidence from the BOXIT Trial. Clin Genitourin Cancer. 2020;18(4):e418–42.
Konala VM, Adapa S, Aronow WS. Immunotherapy in bladder cancer. Am J Ther. 2022;29(3):e334–7.
Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324(19):1980–91.
Khaki AR, Shan Y, Nelson RE, Kaul S, Gore JL, Grivas P, et al. Cost-effectiveness analysis of neoadjuvant immune checkpoint inhibition vs. cisplatin-based chemotherapy in muscle invasive bladder cancer. Urol Oncol. 2021;39(10):732e9–16.
National Library of Medicine. Surveys 2022. https://www.nlm.nih.gov/nichsr/stats_tutorial/section3/mod1_surveys.html. Accessed 28 Oct 2022.
US Department of Veterans Affairs. Comparing VA Versus Non-VA Costs 2023. https://www.herc.research.va.gov/include/page.asp?id=va-vs-non-va. Accessed 5 May 2024.
York Health Economics Consortium. Sensitivity Analysis 2016. https://yhec.co.uk/glossary/sensitivity-analysis/. Accessed 30 May 2024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable, as only secondary data were used.
Consent to participate
Not applicable, as only secondary data were used.
Consent for publication (from patients/participants)
Not applicable, as only secondary data were used.
Code availability
Code was not made available.
Funding
This study was sponsored by Pfizer. Oracle Company conducted the study and was responsible for validating and storing the data.
Conflict of interest
O.C., T.S., R.P., V.D., and J.V. are or were employees of Oracle, which was a paid consultant to Pfizer in connection with the development of this manuscript. A.E., J.B., S.C., A.T., and J.C. are employees of Pfizer, the sponsor of this study.
Author contributions
All authors contributed to the study design and concept, data analysis and review, data interpretation, and manuscript review.
Availability of data and material
In Supplementary Material we list the data sources used and the respective prices of drugs, medical procedures, etc. The raw model data are not made available.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Clark, O., Sarmento, T., Eccleston, A. et al. Economic Impact of Bladder Cancer in the USA. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00512-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00512-8