Abstract
Aim
The aim of this work was to evaluate the cost-effectiveness of faricimab against relevant therapeutic alternatives used in clinical practice for the treatment of diabetic macular oedema (DMO) in the UK.
Methods
A state-transition (Markov) model, with health states based on visual acuity scores and treatment pathways, was developed to conduct cost-utility analysis of faricimab treat and extend (T&E) regimen versus ranibizumab pro re nata (PRN) and aflibercept PRN over a time horizon of 25 years. Comparison against bevacizumab PRN was considered in scenario analysis. Effectiveness data for faricimab was sourced from the pivotal YOSEMITE and RHINE double-blind randomised controlled trials, and from a network meta-analysis for comparators. Costs and (dis)utilities were taken from nationally published sources or literature. The base case included indirect costs (productivity gains, informal care) given the wider impacts of DMO on society. Sensitivity analyses were conducted.
Results
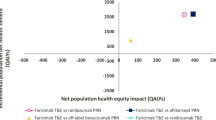
In the base case, faricimab T&E dominated ranibizumab PRN and aflibercept PRN, being more effective and resulting in cost savings (between 0.16 and 0.36 mean QALYs gained, and £5483–9655 mean cost savings). In scenario analysis, faricimab was more effective but costlier compared with bevacizumab, with an incremental cost-effectiveness ratio (ICER) of £8898 per QALY gained. Considering only healthcare payer costs, the ICER of faricimab compared with ranibizumab PRN was £7991 per QALY gained and faricimab dominated aflibercept PRN.
Conclusions
Faricimab T&E has the potential to reduce the burden of vision loss on society, giving people living with DMO greater independence and contributing to increased healthcare system capacity. At a threshold of £20,000, faricimab T&E is cost-effective compared with relevant comparators, and potentially cost saving.
Similar content being viewed by others
At a cost-effectiveness threshold of £20,000, faricimab treat & extend regimen (T&E) is cost-effective compared with flexible treatment regimens used in clinical practice in the UK. |
Faricimab T&E has the potential to reduce the burden of vision loss on society, giving people living with DMO greater independence. |
Given current capacity constraints in the UK health service, the reduced frequency at which faricimab T&E is administered and monitored could be valuable in reducing the burden placed on ophthalmology clinics in the NHS. |
1 Introduction
Diabetic macular oedema (DMO) is a complication of diabetes mellitus, resulting from damage to retinal capillaries in the eye. DMO can result in distortion and blurring of central vision, or in severe cases, irreversible sight loss. It is a leading cause of visual impairment, estimated to affect up to 13% of the 422 million people with diabetes worldwide [1,2,3]. Therefore, in the UK alone, upwards of 500,000 of the nearly 5 million people with a diagnosis of diabetes suffer from DMO, and healthcare resource use associated with DMO is estimated in excess of £100 million [4, 5]. Projections suggest that the prevalence of diabetes could reach 783 million people (uncertainty interval: 605–899 million) globally by 2045 [6]. Demand on ophthalmology services is therefore likely to increase further given that the incidence of DMO is increasing correspondingly.
Anti-vascular endothelial growth factor (anti-VEGF) intravitreal injections are the most common first-line therapy for centre-involving, vision-affecting DMO, which have superseded laser photocoagulation and corticosteroids as the standard of care [7]. In the UK, ranibizumab and aflibercept are anti-VEGF treatments licensed for the treatment of DMO. The National Institute for Health and Care Excellence (NICE) recommends their use in eyes with central retinal thickness of 400 μm or more [8, 9]. Bevacizumab is an additional anti-VEGF sometimes used off-label but does not have marketing authorisation in DMO. Brolucizumab has recently been recommended by NICE, but was not used in the routine treatment of people with DMO in the UK at the time of the analysis [10], and use in routine practice is expected to be limited due to potential safety concerns [11].
Faricimab is a novel bispecific antibody targeting VEGF and angiopoietin-2, an antagonist cytokine of the angiopoietin-1 and Tie2 pathway. DMO is a multifactorial disease characterised by increased permeability of the retinal vasculature; therefore, novel targets beyond the VEGF pathway might promote vascular stability, extend treatment durability, and improve patient outcomes. The angiopoietin (Ang) and tyrosine kinase with immunoglobulin-like and epidermal growth factor homology domains (Tie) signalling pathway is a key regulator of vascular stability, and Ang-2 upregulation has been implicated in the pathogeneses of diabetic macular oedema and other retinal vascular diseases. As such, dual pathway inhibition via Ang-2 and VEGF-A blockade is a novel therapeutic strategy for the treatment of patients with diabetic macular oedema [12].
Phase III double-blind randomised controlled trials (YOSEMITE and RHINE) have demonstrated non-inferiority in change from baseline in visual acuity (BCVA) for faricimab treat and extend (T&E) regimen compared with aflibercept administered every 8 weeks following a loading phase [12]. Faricimab was recently recommended as a first-line option in DMO by NICE [13].
As well as demonstrating clinical effectiveness, cost-effectiveness is an increasingly important part of the evidence base, informing the adoption of new pharmaceuticals both at national and local decision-making levels. A de novo state-transition (Markov) model was constructed to evaluate the cost-effectiveness of faricimab in the UK compared with flexible treatment regimens used in clinical practice. This paper details the model structure, input parameters, results and characterisation of uncertainty.
2 Methods
The model reflects a population of adults with DMO (62 years old, 60% men), 78% of which are anti-VEGF treatment-naïve, as per the intention to treat analysis of the pivotal YOSEMITE and RHINE randomised trials [12]. Base-case analyses compared faricimab treat and extend (T&E) with aflibercept pro re nata (PRN) and ranibizumab PRN. Given bevacizumab is not indicated in DMO but is occasionally used off-label, a comparison was performed as a scenario.
Evidence on the effectiveness of faricimab T&E compared wit relevant comparators was derived from a network meta-analysis (NMA). NMA enabled the effectiveness of faricimab T&E from YOSEMITE and RHINE to be pooled and facilitated indirect comparisons with aflibercept PRN, ranibizumab PRN and bevacizumab PRN.
The model incorporated data on effectiveness, mortality, adverse events, health related quality of life (HRQoL) and costs (direct and indirect) to perform a cost-utility analysis. The analysis took a societal perspective, with a healthcare perspective used in scenario analysis.
2.1 Intervention and Comparators
The modelled intervention and comparators and their respective regimens are summarised in Table 1. Faricimab is administered in a treat and extend regimen with a loading phase followed by flexible treatment intervals. This is likely to be standard of care rather than fixed dose, in line with the pivotal randomised trials. Aflibercept, ranibizumab and bevacizumab are administered every 4 weeks during an initial loading phase and then pro re nata thereafter, as required. These regimens reflect the recommended treatment approaches in the UK, including those from the UK expert panel on the use of aflibercept in the treatment of DMO (referred to as monitor and extend) [14]. An additional T&E ranibizumab comparator is included as a scenario, given that this alternative regimen is sometimes used in UK clinical practice and as data were available for this regimen to enable inclusion in the NMA. Data for high-dose (8 mg) aflibercept were not available at the time of analysis, and it is currently not licensed and not recommended by NICE for use in DMO in the UK.Footnote 1 Although brolucizumab has recently been recommended by NICE, it was not included in the analysis as it is not currently used in the routine treatment of people with DMO in the UK and its use is expected to be limited due to potential safety concerns [11].
2.2 Model Structure
The model structure is depicted in Figs. 1, and 2 and is designed to describe the natural course of the disease and development of DMO. The DMO cohort state-transition model includes a partial relaxation of the Markov assumption, as the model contains time-dependent probabilities (mortality) and treatment-related tunnel states to segment the cohort according to time on/off treatment. State-transition models are well suited for modelling chronic and degenerative diseases, explicitly allowing for time and treatment pathways to appropriately account for differences in costs and quality of life [19]. There is also precedence for the use of similar conceptual model in age-related macular degeneration (AMD), from UK guidelines which included extensive review of the existing literature to inform the modelling approach [20].
The model consists of 6 categories of vision-related health states determined by visual acuity (VA) score (> 85, 85–71, 70–56, 55–41, 40–26 and ≤ 25), where a lower score indicates worse vision (see Fig. 1). The score corresponds to letters correctly identified in a visual acuity test.
The model cohort is distributed across these health states at baseline [see Supplementary Material (Online Resource) Table A1] on the basis of the proportion of patients included in the YOSEMITE and RHINE clinical trials [12]. Each eye is modelled independently, assuming that all patients in the cohort have at least one eye with DMO, and 46.5% have DMO in both eyes at baseline. This was based on previous NICE appraisals and validated in consultation with three UK experts who provided input for all expert clinical inputs [9, 21]. The model differentiates three periods following the clinical pattern typically observed for DMO:
-
Year 1: long loading phase where most vision improvements occur
-
Year 2: characterized by a stabilization of the disease and maintenance of vision gains previously achieved
-
Year 3 and beyond: reduced treatment intensity and long-term maintenance.
The condition of patients in the cohort can be stable, where they remain in the same visual acuity state, or they can transition between states (increased or decreased visual acuity). In Year 1 patients can move up two health states (e.g. VA 40–26 to VA 70–56) and down one health state (e.g. VA 70–56 to VA 55–41). In Year 2 patients can move up or down one health state and in Year 3 patients can move down up to two health states. This was based upon clinical patterns observed in clinical trials with long follow-up, including Protocol T [22], and reflects that patients are unlikely to regain vision in the long term. Non-treatment-related costs (i.e. determined by visual impairment) and utility values are assigned by VA health state category.
The visual acuity health states are combined with the treatment pathway states (see Fig. 2) to appropriately apply costs of treatment (acquisition, administration and monitoring), health state utilities and treatment related disutility values to the proportion of the cohort on/off treatment to reflect the impact of greater vision loss for those who discontinue treatment. The model assumed treatment duration of 5 years, with 15% of patients remaining on long-term treatment, which aligns with a retrospective cohort study [23] and was validated in consultation with UK clinical experts [21]. The dotted line indicates transitions that can only be made for patients with emerging involvement of the second eye.
2.3 Model Input Parameters
The model required estimation of key model input parameters including transition probabilities and treatment effectiveness, health state utility values, adverse events, direct costs and indirect costs.
2.3.1 Effectiveness
Transition probabilities between visual acuity states in the faricimab T&E arm were derived from pooled data from the YOSEMITE and RHINE trials [12] by fitting a multistate model in R programming language (msm package). The best fitting model contained four states [see Supplementary Material, (Online Resource) Fig. A2], which were defined corresponding to a transition of one or two VA state increase, one VA state decrease or no transition: state 1, gain of at least 22.5 letters; state 2, gain of between 7.5 and 22.5 letters; state 3, between gain of 7.5 and loss of 7.5 letters (i.e. stable); and state 4, loss of 7.5 or more letters. In the first year, transitions depend on baseline visual acuity score given it is an important prognostic factor in treatment effectiveness. Thereafter, transitions are independent of baseline score.
Effectiveness data for model comparators was informed by an NMA, described in full elsewhere [24]. In brief, a systematic literature review was performed to identify randomised controlled trials containing relevant comparators (including placebo/sham to form networks) in patients with DMO older than 18 years. Outcomes include changes in baseline visual acuity, change in retinal thickness, injection frequency and adverse events. The NMA showed that faricimab T&E demonstrated superior or comparable efficacy in terms of visual acuity outcomes compared with the relevant comparator anti-VEGF treatment regimens for DMO after 1 year [25]. Therefore, effectiveness for year 1 is based on YOSEMITE and RHINE for faricimab T&E and the NMA for all comparators. Beyond year 1, the second year treatment effect derived from YOSEMITE and RHINE for faricimab T&E is applied to all interventions during treatment [22].
Background mortality was informed by UK life tables, using data from Office for National Statistics for years 2017–2019, adjusted for increased mortality amongst a diabetic population and the impact of visual impairment [20, 26, 27].
2.3.2 Adverse Events
Treatment-related adverse event probabilities were informed by YOSEMITE and RHINE for faricimab T&E and applied for all interventions on the basis of the finding from the NMA of comparable adverse event results. Costs and disutility were taken from a UK guideline in nAMD [20] and are shown in Supplementary Material (Online Resource) Table A3.
2.3.3 Costs
Costs were presented in 2019/2020 prices and were made up of both direct healthcare payer costs to the UK National Health Service (NHS) and Personal Social Services (PSS), including ongoing supportive care, as well as indirect costs of DMO treatment (productivity impacts and informal care costs). Travel costs were not included given their negligible impact. In the base-case these wider personal and societal costs were included given their importance in relation to the impact of DMO on patients and society. A 2018 UK study estimated indirect costs of sight loss due to AMD, cataract, diabetic retinopathy, glaucoma and under-corrected refractive error of £5.65 billion, which was nearly double the value of estimated direct healthcare costs [28]. A scenario analysis was also presented considering only NHS costs.
Acquisition costs (see Table 1) were taken from list prices available from the British National Formulary [15,16,17,18]. The proportion of patients receiving faricimab at 4, 8, 12 or 16 weekly doses after the loading phase in years 1 and 2 was informed by YOSEMITE and RHINE pooled data [12]. It was assumed that no additional monitoring is required between treatment visits during years 1 and 2 as per T&E regimen.
Frequency of administration for PRN regimens in year 1 was informed by the NMA [24], with the number of injections apportioned over the year. In year 2 and beyond, administration frequency and monitoring visits throughout treatment was informed by a randomised trial of the three comparator treatments and an extension cohort study (Protocol T) of the same group of patients. The same source was used to inform monitoring in the faricimab T&E arm after 2 years, where patients are likely to move to PRN regimen. To account only for additional monitoring costs, this was implemented in the model as the difference between number of visits and number of treatments. The resultant mean frequency of administration and monitoring visits are presented in Supplementary Material (Online Resource) Table A4, alongside the annual acquisition costs in year 1 to year 3 for each treatment.
Administration costs (see Table 2) were informed by a UK clinical guideline and a NICE technology appraisal in nAMD [20, 29]. Costs were composed of a consultant-led outpatient attendance, an optical coherence tomography (OCT) and a cost of the injection, where unit costs were informed by the National Schedule of NHS costs [30].
Supportive care costs (Table 2) were sourced from a UK guideline and were composed of low vision and blindness resources which fall upon NHS and Personal Social Services (vision-related depression, low vision rehabilitation, hip replacement due to falls, residential and community care) and wider societal costs (housing and council tax benefit, social security and governmental tax allowances) [20]. Health states that encompass at least one eye with visual acuity of 55 letters or less incur costs associated with visual impairment. Cost associated with blindness are assigned to health states where both eyes have visual acuity of 25 letters or less. Costs were inflated to 2019/2020 costs using published inflation indices [31].
The costs of productivity losses/gains (see Table 3) were estimated using a human capital approach. Costs were computed on the basis of remaining productive working life years determined by the statutory state pension age (66 years), productivity for relative visual impairment and working years valued at the UK average market wages [34, 35].
Informal care costs for visual impairment were informed by data from a 2018 study in Portugal, which assessed informal care associated with vision loss [39]. Marques et al. reported a mean of 470 h per annum for the 39.6% of all interviewees who received some informal care. Therefore, informal care hours of 186 h per year (39.6% × 470) were valued at UK average market wages [35]. The costs of informal carers taking time off work for treatment and monitoring visits were assumed to be 4 h, valued in the same way.
2.3.4 Utilities
Utility values (see Table 4) are based on a study by Czoski-Murray et al. conducted in the UK which estimated a regression model to relate visual acuity score and utility on the basis of time trade-off methods, including an age covariate [40]. A further adjustment was applied to account for better vision in one eye, partially compensating for worse vision in the other. Aligned with the approach in a guideline review conducted by NICE in nAMD [20], a scaling factor of 0.3 is used to estimate the impact of changes in the worse seeing eye on utility. A disutility for injections and adverse events was included in the model. It was assumed that 50% of patients experience a total loss of quality of life on treatment days, informed by the same UK guideline [20].
2.3.5 Cost-Effectiveness Analysis
The state transition model and cost-utility analyses were operationalised in Microsoft Excel©. The model adopted a 4-week cycle length, which is typically the shortest treatment period observed in clinical practice. Given the short cycle length, the impact of event timings within this period were considered negligible and therefore no half-cycle correction was applied. A discount rate of 3.5% was applied to costs and utilities according to UK guidelines [41]. Analyses were run for cohort aged 62 years and with a time horizon of 25 years to ensure important differences in costs and effects were captured. Results were estimated for difference in quality adjusted life-years (QALYs), costs and net monetary benefit (NMB), with health benefits valued using the lower end of cost-effectiveness threshold adopted by NICE (£20,000 per QALY) [41]. Base-case results were based on a societal perspective and presented deterministically.
Deterministic and probabilistic sensitivity analysis (PSA, 1000 simulations) were conducted to characterise uncertainty and explore robustness of model results. Upper and lower bounds for deterministic sensitivity analysis (DSA) were informed by source material estimates of uncertainty, 95% confidence interval where available, or an arbitrary 20% of the mean value otherwise to reflect limited knowledge of uncertainty around mean values, and incremental NMB was used as the output. The distributions used in the PSA were based on parameter characteristics, and distributional shape was defined by observed data (i.e. alpha, beta, mean, standard error). The PSA inputs for visual acuity changes, treatment discontinuation and number of injections were implemented on the basis of sampling with replacement from the empirical distribution for trial-based data, or CODA outputs from the Bayesian NMA for comparators. Cost parameters used gamma distributions; proportions, probabilities and utility values used beta distributions. A normal distribution was used for all other parameters. A full table of model inputs not derived from NMA is given in Supplementary Material (Online Resource) Table A4, including characterisation of uncertainty. A healthcare payer perspective was presented in a scenario, as well as a comparison with bevacizumab and ranibizumab T&E. Alternative health state utilities from a study by Brown and colleagues were presented in scenario [see Supplementary Material (Online Resource) Table A6], which were similarly used as sensitivity analysis in a recent NICE guideline [20, 42].
3 Results
Base-case results comparing faricimab T&E against ranibizumab PRN and aflibercept PRN over a lifetime are presented in Table 5. For both comparisons, faricimab T&E produced additional mean life years (LY) and QALYs, with most of the benefit captured in quality-of-life improvements. The mean benefit of faricimab T&E over comparators ranged from 0.16 to 0.36 QALYs. Faricimab T&E resulted in cost savings compared with ranibizumab PRN (£5483) and aflibercept PRN (£9655) and is therefore considered dominant.
A breakdown of the components of costs is given in Table 6. Drug acquisition, informal care and cost offsets from productivity gains are the largest components of the total cost estimates. Given that fewer monitoring visits are required, monitoring costs for faricimab T&E are approximately 50% of the other comparators (£1858 versus £4165 and £4325). Productivity gains from faricimab T&E compared with ranibizumab PRN and aflibercept PRN were £4824 and £2388, respectively. Faricimab T&E informal care costs (£22,341) were lower than ranibizumab PRN (£25,854) and aflibercept PRN (£24,110).
4 Sensitivity and scenario analysis
Deterministic sensitivity analysis is summarised in the tornado diagrams shown in Figs. 3 and 4, which display the ten most sensitive parameters included in the analysis for comparisons against aflibercept PRN and ranibizumab PRN, respectively. Results were most sensitive to the starting age of the cohort, drug costs and productivity input parameters. All analyses suggested positive incremental NMB for faricimab T&E versus comparators.
Results of the probabilistic sensitivity analysis, jointly characterising uncertainty in all input parameters, are presented using cost-effectiveness acceptability curves (CEAC). It shows the probability of each regimen being the most cost-effective option at willingness to pay of £0 to £50,000 (see Fig. 5). Faricimab T&E has the highest likelihood of being the most cost-effective option across the range. At a willingness-to-pay threshold of £20,000 per QALY, the probability that faricimab T&E is cost-effective compared with aflibercept PRN and ranibizumab PRN is 85%. At lower thresholds ranibizumab PRN displays a small probability of being most cost-effective (£5000: 24%; £10,000: 19%)
Results of scenario analyses comparing faricimab T&E against bevacizumab PRN and ranibizumab T&E and incorporating healthcare resource use costs only are presented in Tables 7 and 8, respectively. Results of the scenario analysis exploring alternative health state utility values by Brown and colleagues are presented in Table 9 [42]. Mean costs per patient are lower for bevacizumab PRN (£3478) and mean QALYs are also lower (− 0.39) compared with faricimab T&E, resulting in an ICER of £8898 per QALY gained or incremental net benefit (INB) of £4339, using cost-effectiveness threshold of £20,000 per QALY.
When only NHS costs are included, faricimab T&E still dominates aflibercept PRN (see Table 8). Patients in the faricimab T&E arm accrued more costs compared with ranibizumab PRN. However, given the QALY benefits, faricimab T&E would be considered cost-effective at a threshold of £20,000 per QALY compared with ranibizumab PRN.
5 Discussion
National and local decision-makers are increasingly concerned with cost-effectiveness and the cost-saving potential of new interventions. A de novo model was constructed to conduct a UK cost-utility analysis of faricimab T&E compared with relevant alternatives. Analyses demonstrate that faricimab T&E is cost saving or cost-effective compared with aflibercept PRN, ranibizumab PRN and T&E and bevacizumab PRN at a cost-effectiveness threshold of £20,000 per QALY. Probabilistic analysis demonstrated that there is a high degree of certainty that faricimab T&E is cost-effective compared with ranibizumab PRN and aflibercept PRN. Scenario analysis versus bevacizumab PRN, which is not licensed for the treatment of DMO in the UK but occasionally used in clinical practice, suggests that although bevacizumab PRN is less costly, faricimab T&E would be considered a cost-effective option due to greater health gains.
Deterministic sensitivity analysis suggested that the age of cohort was the most sensitive parameter. This suggests that reducing the starting of age of treatment, for example, by improved diagnosis, could further increase the net monetary benefit from faricimab T&E substantially. This is driven by the productivity gains from the younger patient population and the relatively large bounds explored (ages 42 and 82 years). Furthermore, the analysis conservatively assumes an average retirement age of 65 years for the cohort. Given that the state pension age in the UK is set to rise in the future, the period over which productivity gains are estimated will also increase, leading to further increases in productivity gains and net monetary benefit.
The reduction of independence has been found to be a major concern for people with diabetic retinopathy, including people with DMO, with limitations in social activities and driving having a substantial impact [43]. Base-case analyses incorporating informal care costs and productivity gains suggest faricimab T&E produces cost savings in addition to improvements in quality of life against ranibizumab PRN and aflibercept PRN. These indirect costs have been shown to be an important part of the burden of DMO, in addition to the direct healthcare costs to the NHS [4, 28]. When only NHS and PSS costs are considered, faricimab T&E can still be considered cost-effective. In addition, administration and monitoring costs were lower for faricimab T&E than all alternatives.
Whilst several HTA agencies typically take a narrow healthcare perspective, there are other societal and healthcare impacts that provide value but are not routinely included in HTA decision-making. For health services that have significant capacity constraints, such as ophthalmologic clinics, effective treatments which reduce healthcare resource requirements can alleviate some of these capacity issues [44]. Ophthalmology is the busiest outpatient speciality in the NHS [45] and there is a severe shortage of ophthalmologists to manage the increasing demand for services. This is likely to increase further given that the incidence of type 1 and type 2 diabetes is increasing, and the incidence of DMO is increasing correspondingly [28, 46]. The coronavirus disease 2019 (COVID-19) pandemic further exacerbated the strain on ophthalmic services; ophthalmology outpatient attendances in the UK fell from 7.9 million in 2019/2020 to 5.4 million in 2020/2021, a drop of 32% [45, 47]. This created a large backlog for ophthalmic care in the UK, with more than 600,000 patients awaiting ophthalmology treatment as of December 2021, including 27,000 patients who have been waiting for a year or more [48]. The reduced frequency at which faricimab T&E is administered and monitored offers a potential route for alleviating part of the burden on ophthalmology services [49].
This study presents the first model of its kind comparing faricimab for the treatment of DMO against relevant comparators and regimens in a UK setting, with faricimab effectiveness informed by pooled analysis of two large randomised controlled trials [12]. The model structure, and some of the key assumptions having precedence and clinical validation, have been used to inform UK national guidelines in a similar disease area [20]. Key input parameters, including comparator effectiveness and frequency of administration, were sourced from an NMA performed following a systematic review of the literature.
5.1 Limitations
There is some variation in clinical practice and adaptations in the use of treatments for DMO, with a general move away from PRN to T&E regimens. Analyses were conducted in line with pivotal trials YOSEMITE and RHINE and on the basis of UK clinical expert opinion on the use of comparator treatments. Analyses against other potential regimens using fixed monthly or bimonthly treatment could be performed, but were deemed of limited relevance in UK clinical practise as outlined above. [21] Similarly, analysis against brolucizumab was not included given the respective NICE appraisal highlighting that it may rather be used as a second-line treatment [10]. State-transition (Markov) models are appropriate for modelling chronic conditions and there is significant precedence for use in DMO [20]. However, other approaches, including individual patient simulation, may be able to better capture patient heterogeneity and have been explored in ophthalmology applications [50].
Direct clinical evidence was not available to compare faricimab T&E with ranibizumab and bevacizumab regimen. For these comparators, data were informed by a Bayesian NMA of 26 studies [24]. This analysis suggests faricimab is superior to flexible dosing regimens on the basis of retinal thickness, and superior or equal in terms of BVCA (credible intervals include 0 only for aflibercept), with injection frequency numerically lower, reflecting some remaining uncertainty. However, these Bayesian methods are consistent with probabilistic sensitivity analysis, allowing this uncertainty to be jointly characterised by sampling with replacement from the empirical distribution or NMA CODA. PSA showed a high probability that faricimab is the most cost-effective option.
Health state utility values for visual conditions such as DMO pose some difficulties, with concerns about the performance of commonly recommended generic measures such as the EQ-5D [51]. The source for utility values was a study that applied a novel method by recreating visual acuity groups, using contact lenses and performing direct elicitation [33]. These visual acuity groups were able to directly map the health states used in the model. There has been criticism of the results of this approach, suggesting that it may underestimate the disutility associated with loss in visual acuity [52]. This may have the impact of consequently also underestimating the benefits of faricimab T&E relative to the comparator treatment regimens. A scenario analysis suggested results were not sensitive to use of alternative utility values.
All analyses were based on list prices. In practice, treatments may be offered in the UK at discounted prices, due to patient access schemes negotiated in parallel to NICE Technology Assessments. In addition, a vial of bevacizumab formulated for cancer treatment may be compounded by pharmacies into multiple doses for off-label intravitreal treatment. However, acquisition costs varied by ± 10% in sensitivity analysis and results were robust to changes.
6 Conclusion
Faricimab T&E can be considered cost-effective treatment for DMO compared with ranibizumab PRN and T&E, aflibercept PRN and bevacizumab PRN, whether using a societal or healthcare payer perspective for costs. The deterministic and probabilistic sensitivity analysis performed suggests results are robust to allowance for uncertainty.
Faricimab as a new treatment option has the potential to reduce the burden of vision loss on patients and on society, giving people living with DMO greater independence. Further, given the current capacity constraints in the health system and the expected increase in future demand for ophthalmology services, the reduced frequency at which faricimab T&E is administered and monitored could be valuable in reducing the burden placed on ophthalmology clinics in the NHS.
Notes
Data on the effectiveness of the T&E regimen using aflibercept in treatment-naïve patients were not available for inclusion in the NMA.
References
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
WHO. Diabetes: World Health Organization; [cited 2022 Sep 21]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/diabetes.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64.
Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol. 2012;96(3):345–9.
Whicher CA, O’Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020;37(2):242–7.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
Wenick AS, Bressler NM. Diabetic macular edema: current and emerging therapies. Middle East Afr J Ophthalmol. 2012;19(1):4–12.
NICE. Overview | Ranibizumab for treating diabetic macular oedema: National Institute for Health and Care Excellence [cited 2022 Sep 21]. Available from: https://www.nice.org.uk/guidance/ta274.
NICE. Overview | Aflibercept for treating diabetic macular oedema: National Institute for Health and Care Excellence; [cited 2023 March 22]. Available from: https://www.nice.org.uk/guidance/ta346.
NICE. Overview | Brolucizumab for treating diabetic macular oedema: National Institute for Health and Care Excellence [cited 2022 Dec 13]. Available from: https://www.nice.org.uk/guidance/ta820.
DH. Department of Health for England: Brolucizumab (Beovu▼): risk of intraocular inflammation and retinal vascular occlusion increased with short dosing intervals 2022. Available from: https://www.gov.uk/drug-safety-update/brolucizumab-beovuv-risk-of-intraocular-inflammation-and-retinal-vascular-occlusion-increased-with-short-dosing-intervals.
Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399(10326):741–55.
NICE. Faricimab for treating diabetic macular oedema (TA799). Technology appraisal guidance: National Institute for Health and Care Excellence [cited 2022 Sep 23]. Available from: www.nice.org.uk/guidance/ta799.
Pearce I, Bailey C, Fletcher E, Ghanchi F, Rennie C, Santiago C, et al. Translating evidence into practice: recommendations by a UK expert panel on the use of aflibercept in diabetic macular oedema. Eye (Lond). 2020;34(5):969–81.
BNF. Medicinal forms | Faricimab | Drugs British National Formulary [cited 2022 Sep 21]. Available from: https://bnf.nice.org.uk/drugs/faricimab/medicinal-forms/.
BNF. Medicinal forms | Aflibercept | Drugs | BNF British National Formulary [cited 2022 Sep 21]. Available from: https://bnf.nice.org.uk/drugs/aflibercept/medicinal-forms/#solution-for-injection.
BNF. Medicinal forms | Ranibizumab | Drugs | BNF British National Formulary [cited 2022 Sep 21]. Available from: https://bnf.nice.org.uk/drugs/ranibizumab/medicinal-forms/.
BNF. Medicinal forms | Bevacizumab | Drugs | BNF British National Formulary [cited 2022 Sep 21]. Available from: https://bnf.nice.org.uk/drugs/bevacizumab/medicinal-forms/.
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–310.
NICE. NG82 Macular degeneration. Appendix J: Health economics: National Institute for Health and Care Excellence 2018 [cited 2022 Sep 23]. Available from: https://www.nice.org.uk/guidance/ng82/evidence/appendix-j-health-economics-pdf-170036251093.
NICE. TA799 Company evidence submission. Faricimab for treating diabetic macular oedema: National Institute for Health and Care Excellence 2022 [cited 2022 Dec 14]. Available from: https://www.nice.org.uk/guidance/ta799/evidence/committee-papers-pdf-11127510397.
Glassman AR, Wells JA 3rd, Josic K, Maguire MG, Antoszyk AN, Baker C, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020;127(9):1201–10.
Kern C, Fu DJ, Huemer J, Faes L, Wagner SK, Kortuem K, et al. An open-source data set of anti-VEGF therapy in diabetic macular oedema patients over 4 years and their visual acuity outcomes. Eye (London). 2021;35(5):1354–64.
Watkins C, Paulo T, Bührer C, Holekamp NM, Bagijn M. Comparative efficacy, durability and safety of faricimab in the treatment of diabetic macular edema: a systematic literature review and network meta-analysis. Adv Ther. 2023:1-18.
Régnier S, Malcolm W, Allen F, Wright J, Bezlyak V. Efficacy of anti-VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: a systematic review and network meta-analysis. PLoS One. 2014;9(7): e102309.
Christ SL, Lee DJ, Lam BL, Zheng DD, Arheart KL. Assessment of the effect of visual impairment on mortality through multiple health pathways: structural equation modeling. Invest Ophthalmol Vis Sci. 2008;49(8):3318–23.
ONS. National life tables – life expectancy in the UK - Office for National Statistics: Office for National Statistics; [cited 2022 Sep 21]. Available from: ttps://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2017to2019.
Pezzullo L, Streatfeild J, Simkiss P, Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18(1):63.
NICE. Evidence Review Group Report | Aflibercept solution for injection for treating wet age‑related macular degeneration | Guidance | NICE: National Institute for Health and Care Excellence [cited 2022 Sep 21]. Available from: https://www.nice.org.uk/guidance/ta294/history.
NHSE. National Schedule of NHS costs. 2019/20 National Cost Collection Data Publication: NHS England; [cited 2022 Sep 21]. Available from: https://www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/.
Jones KB, A. Costs of Health and Social Care 2021 2021 [cited 2022 Oct 24]. Available from: https://kar.kent.ac.uk/92342/25/Unit Costs Report 2021 - Final version for publication %28AMENDED2%29.pdf.
CADTH. CADTH Reimbursement Review: Faricimab (Vabysmo). 2023.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–9.
UKDWP. State Pension age timetable - GOV.UK: UK Department for Work and Pensions; [cited 2022 Sep 21]. Available from: https://www.gov.uk/government/publications/state-pension-age-timetable/state-pension-age-timetable.
ONS. Annual Survey of Hours and Earnings time series of selected estimates: Office for National Statistics; 2021 [cited 2022 Sep 21]. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/ashe1997to2015selectedestimates.
ONS. A01: Summary of labour market statistics: Office for National Statistics; [cited 2022 Sep 21]. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/summaryoflabourmarketstatistics.
ONS. Disability and employment Office for National Statistics; [cited 2022 Sep 21]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/disability/datasets/disabilityandemployment.
Slade JE, E; Crawley, T; (Royal National Institute of Blind People). Employment for blind and partially sighted people in 2019 - RNIB 2022 [cited 2022 Sep 21]. Available from: https://www.rnib.org.uk/sites/default/files/Employment for blind and partially sighted people in 2019 - External version.docx.
Marques AP, Macedo AF, Hernandez-Moreno L, Ramos PL, Butt T, Rubin G, et al. The use of informal care by people with vision impairment. PLoS One. 2018;13(6): e0198631.
Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health. 2009;12(5):793–9.
NICE. NICE health technology evaluations: the manual. Processes and methods: National Institute for Health and Care Excellence 2022 [cited 2022 Sep 21]. Available from: https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741.
Brown GC, Sharma S, Brown MM, Kistler J. Utility values and age-related macular degeneration. Arch Ophthalmol. 2000;118(1):47–51.
Coyne KS, Margolis MK, Kennedy-Martin T, Baker TM, Klein R, Paul MD, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21(4):447–53.
Gale R, Cox O, Keenan C, Chakravarthy U. Health technology assessment of new retinal treatments; the need to capture healthcare capacity issues. Eye (Lond). 2022;36(12):2236–8.
NHSDigital. Data on file: Hospital Outpatient Activity 2020-21: All Attendances, (September 2020). 2020.
ONS. Office for National Statistics population projections: Office for National Statistics; 2018. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/z3zippedpopulationprojectionsdatafilesengland
NHSDigital. Data on file: Hospital Outpatient Activity 2019-20: All Attendances, (October 2020). 2020.
NHSE. Data on file: Consultant-led Referral to Treatment Waiting Times Data 2021-22, Incomplete Commissioner Dec21 (February 2022) NHS England; Available from: https://www.england.nhs.uk/statistics/statistical-work-areas/rtt-waiting-times/rtt-guidance/.
NICE. Supporting the health and care system in improving productivity 2023 [cited 2023 5 May]. Available from: https://www.nice.org.uk/productivity.
Claxton L, Hodgson R, Taylor M, Malcolm B, Pulikottil JR. Simulation modelling in ophthalmology: application to cost effectiveness of ranibizumab and aflibercept for the treatment of wet age-related macular degeneration in the United Kingdom. Pharmacoeconomics. 2017;35(2):237–48.
Tosh J, Brazier J, Evans P, Longworth L. A review of generic preference-based measures of health-related quality of life in visual disorders. Value Health. 2012;15(1):118–27.
Butt T, Crossland MD, West P, Orr SW, Rubin GS. Simulation contact lenses for AMD health state utility values in NICE appraisals: a different reality. Br J Ophthalmol. 2015;99(4):540–4.
Acknowledgements
Third-party writing assistance was provided by Matthew Glover and Louise Longworth of Putnam PHMR. This writing assistance was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding was provided by F. Hoffmann-La Roche Ltd. for third-party writing assistance, which was provided by Matthew Glover and Louise Longworth of Putnam PHMR. Funding for open access was provided by F. Hoffmann-La Roche Ltd.
Conflicts of Interest
Christian Bührer is employed by and reports stock ownership in F. Hoffmann-La Roche Ltd. Tatiana Paulo and Marloes Bagijn are employees of F. Hoffmann-La Roche Ltd. Thomas Paling is an employee of Roche Products Ltd. Richard Gale has received financial support from consultancy support from Hoffmann-La Roche Ltd, Novartis and Bayer.
Availability of Data and Material
All data used for this study are provided in the article or are publicly available as indicated by citations.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
The cost-effectiveness model was developed in Microsoft Excel 365 (Microsoft Corporation). Additional information about model programming is available from the corresponding author upon request.
Author Contributions
Conceptualization: Christian Bührer, Thomas Paling, Tatiana Paulo and Marloes Bagijn; methodology: Christian Bührer, Thomas Paling, Tatiana Paulo and Marloes Bagijn; formal analysis and investigation: Christian Bührer, Thomas Paling, Richard Gale, Tatiana Paulo and Marloes Bagijn; writing—review and editing: Christian Bührer, Thomas Paling, Richard Gale, Tatiana Paulo and Marloes Bagijn; funding acquisition: Christian Bührer. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bührer, C., Paling, T., Gale, R. et al. Cost-Effectiveness of Faricimab in the Treatment of Diabetic Macular Oedema (DMO): A UK Analysis. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-023-00465-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-023-00465-4