Abstract
Objective
There are no publications that have demonstrated economic value for ankylosing spondylitis (AS) treatments in Indonesia. Cost per responder (CPR) is a lean method of economic evaluation. We estimated CPR from Indonesia’s health system perspective following AS treatment with secukinumab relative to adalimumab, golimumab, and infliximab.
Methods
In the absence of head-to-head trials, a comparative evidence analysis was conducted in the form of matching-adjusted indirect comparison (MAIC) to estimate the response rate of various competing treatment options against secukinumab. This was followed by a CPR analysis that compared the cost per patient for a defined response level.
Results
Based on MAIC, patients on secukinumab had higher Assessment in Spondyloarthritis International Society (ASAS) 20 response (improvement of ≥ 20% and ≥ 1 unit in at least three domains on a scale of 10 and no worsening of ≥ 20% and ≥ 1 unit in remaining domain on a scale of 10) and ASAS 40 response (improvement of ≥ 40% and ≥ 2 units in at least three domains on a scale of 10 and no worsening at all in remaining domain) versus those on adalimumab, golimumab, and infliximab at week 24. The cost per ASAS 20 at week 24 for secukinumab was 75% lower than adalimumab, 65% lower than golimumab, and 80% lower than infliximab. The cost per ASAS 40 at week 24 for secukinumab was 77% lower than adalimumab, 67% lower than golimumab, and 83% lower than infliximab. Secukinumab dominated adalimumab, golimumab, and infliximab at week 24 and adalimumab at week 52, by being more efficacious at lower cost. Threshold analysis revealed that substantial reduction in efficacy or increase in cost of secukinumab would make secukinumab not cost effective, indicating the robustness of the results.
Conclusion
This study demonstrated that if AS patients in Indonesia were treated with secukinumab instead of comparator therapies, more patients could be treated, and more patients would reach response to treatment for the same budget.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ankylosing spondylitis (AS) patients on treatment with secukinumab had better clinical efficacy compared with other biologics at a lower cost. |
For the same budget, more AS patients in Indonesia can be treated effectively with secukinumab compared with other approved biologics. |
1 Introduction
Ankylosing spondylitis (AS) is an immune-mediated chronic inflammatory disease belonging to a group of conditions known as spondyloarthritis. It is mainly characterized by the involvement of the axial skeleton and sacroiliac joints but also affects the peripheral joints and extra-articular organs. The mean prevalence of AS was 16.7 per 10,000 in Asia, which was lower than that in Europe and North America, but higher than in Latin America and Africa [1].
The Indonesian Rheumatology Association has recently published guidelines for management of AS in Indonesia [2]. The recommended treatment includes nonsteroidal anti-inflammatory drugs (NSAIDs), tumor necrosis factor inhibitors (TNFi), and secukinumab. Secukinumab is a fully human monoclonal antibody to interleukin (IL)-17A. IL-17 antagonism represents a novel therapeutic approach aimed at interference with the chronic inflammatory process by selectively targeting the predominant proinflammatory cytokine of the helper T17 cell subset.
NSAIDs are recommended as the first-line therapy in patients with active AS. TNFi are recommended as the second-line therapy, with no specific preference for any TNFi, and in situations where it is not possible to prescribe TNFi, secukinumab can be given as first-line biologic. Secukinumab is recommended over treatment with a different TNFi in patients with primary non-response to TNFi [2].
Along with the clinical value of a therapy, the economic value should also be demonstrated. Cost per responder (CPR) is one of the methods for demonstrating the economic value. CPR analysis quantifies the relative value of at least two drugs by comparing the cost per patient relative to achieving a defined response level. CPR is a lean economic approach to healthcare resource prioritization that does not replace the widely accepted and more sophisticated health economic evaluation methods. Instead, it is a complementary analysis that can facilitate decision making [3]. However, there are no publications on economic analysis of AS in Indonesia.
The current analysis estimates and compares the CPR from Indonesia’s health system perspective following treatment of AS with secukinumab relative to other comparators, including adalimumab, golimumab, and infliximab.
2 Methods
In the absence of head-to-head clinical trials that directly compare different biologic agents, comparative efficacy assessments have mostly been conducted worldwide using indirect comparison methods. Among indirect comparison methods, matching-adjusted indirect comparison (MAIC) is a robust method. Unlike standard indirect comparison methods, MAIC allows the following to be carried out: (i) similar patient populations can be compared by matching baseline characteristics and (ii) long-term trial results can be compared after patients have switched from placebo (or an active treatment) to the drug of interest even though there is no longer a common comparator [4].
MAIC was conducted to estimate the response rate of various competing treatment options against secukinumab, followed by a CPR analysis that compared the cost per patient for a defined response level.
2.1 Matching-Adjusted Indirect Comparison (MAIC)
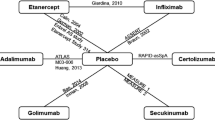
The MAIC method adjusts for differences in patient characteristics at baseline by using individual patient data (IPD) from a trial of one treatment to match the baseline aggregate characteristics reported in a trial of another treatment [4, 5]. By reweighting patient data, MAIC targets comparison of treatment efficacy in the matched population, reducing the impact of heterogeneity between the study populations. After matching, treatment outcomes are compared across balanced trial populations. In the current analysis, IPD data from a secukinumab trial were matched with the adalimumab, golimumab, and infliximab trial data. A MAIC for secukinumab versus adalimumab and golimumab has already been published [4, 5]. The pivotal clinical trials included in the MAIC were MEASURE 1 (ClinicalTrials.gov identifier: NCT01358175), MEASURE 2 (NCT01649375), ATLAS (NCT00085644), GO-RAISE (NCT00265083), and ASSERT (NCT00207701) [4, 5]. Secukinumab had an IV loading dose followed by an SC dose in MEASURE 1 and SC as loading dose followed by an SC dose in MEASURE 2 [6]. At the time of conducting the MAIC, these were the most recent trials of the respective drugs reporting the outcomes of interest at desired time points. All the matching criteria and outcomes considered are reported in Table 1.
The outcomes of the MAIC analysis for each of the comparators were achieved by matching the standard set of variables, which included age, sex, C-reactive protein (CRP) levels, Bath Ankylosing Spondylitis Functional Index (BASFI), and TNF-naive population (standard criteria). The following additional variables were considered for individual comparators:
-
Adalimumab: Standard criteria.
-
Golimumab: Standard criteria and disease duration.
-
Infliximab: Standard criteria, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and disease duration.
MAIC is an accepted technique used by health technology assessment agencies, such as the United Kingdom’s National Institute for Health and Care Excellence (NICE), in their decision-making processes [7,8,9].
2.2 Cost-per-Responder Analysis
A CPR analysis was conducted. To conduct the CPR analysis, model inputs were estimated from publicly available sources, published literature on prescribing information, and Indonesian expert opinions.Footnote 1 We also conducted threshold analysis to assess the impact of various factors on delta CPR.
2.3 Model Inputs
The model utilized the following inputs:
-
1.
Population: The model used the population of adult patients with AS who were eligible to receive biologic treatment based on epidemiological inputs. The inputs were
-
2.
Percentage of AS population treated with biologics from the pool of biologic-eligible AS patients: 0.4% (Indonesian key opinion leader input).
-
3.
Dosing: All approved biologics in the country that are to be used as the treatment option for treating adult patients with AS have been included in the model per their respective label [12,13,14,15] (Table 2).
-
4.
Costs: Two types of costs were considered in this model—drug acquisition costs and administration costs. Costs included in the study are 2021 values.
-
a.
Drug acquisition cost The list price of each treatment option was considered in the analysis. The cost per dose was provided by the Novartis Indonesia team in Indonesian rupiah (IDR). Cost refers to list price in retail market excluding value added tax (Table 2).
-
b.
Administration costsFootnote 2 Drug administration costs were included in the model based on the mode of administration: intravenous (IV) or subcutaneous (SC). These costs were applicable whenever a drug was administered to the patient. IV: IDR1,000,000; SC: IDR300,000.
-
5.
Response rate: Response rates of each comparator were included in the model for the following outcome measures: Assessment in Spondyloarthritis International Society (ASAS) 20 (improvement of ≥ 20% and ≥ 1 unit in at least three domains on a scale of 10 and no worsening of ≥ 20% and ≥ 1 unit in remaining domain on a scale of 10) , ASAS 40 response (Improvement of ≥ 40% and ≥ 2 units in at least three domains on a scale of 10 and no worsening at all in remaining domain), BASDAI 50 (50% improvement of the initial BASDAI score). Response rates were estimated at weeks 24 and 52 based on MAIC. The ASAS 20 and ASAS 40 response rates based on MAIC have been previously published for secukinumab versus adalimumab and secukinumab versus golimumab [4, 5]. BASDAI 50 response rates based on MAIC have not been published to date, and are reported in the electronic supplementary material (ESM).
2.4 Model Outcomes
Based on the model inputs, the following outcomes have been reported:
-
1.
CPR The CPR for each treatment for a given outcome was estimated by dividing the total cost of a particular treatment course by the corresponding response rate (Fig. 1).
CPR estimates the cost for one successful responder for a given treatment over a given time horizon.
-
2.
Number needed to treat (NNT): This is an estimate of the number of patients who need to be treated to have an additional responder.
$$\mathrm{NNT }=\frac{1}{\mathrm{Secukinumab responder rate}-\mathrm{Comparator responder rate}}.$$ -
3.
Incremental costs per additional responder (ICAR): This is the incremental cost of treatment to obtain an additional responder versus a comparator.
$$\mathrm{ICAR }=\frac{\mathrm{Total cost of secukinumab}-\mathrm{Total cost of comparator }}{\mathrm{Secukinumab responder rate}-\mathrm{Comparator responder rate}}.$$
2.5 Threshold Analysis (TA)
TA provides a cut-off point for a parameter at which the results change in the opposite direction. For the current analysis, TA was conducted on ASAS 20 and ASAS 40 for secukinumab, adalimumab, golimumab, and infliximab at week 24. The following parameters were tested in TA one by one: percent response (secukinumab vs comparator), cost of secukinumab, and cost of comparator. The value at which the difference between CPR for secukinumab versus comparator became 0 was considered as the threshold point.
Ethical approval for the study was not obtained as the clinical trial data used for the analysis was already available in public domain.
3 Results
3.1 MAIC
Patients on secukinumab had higher ASAS 20 response versus those on adalimumab, golimumab, and infliximab at week 24 (Fig. 2). The response was similarly higher at week 52 (secukinumab vs adalimumab,74% vs 65; secukinumab vs infliximab, 92% vs 69%).
ASAS 20 and ASAS 40 response at week 24. Standard matching criteria for all comparators: age, sex, C-reactive protein (CRP) levels, BASFI, and TNF-naive population. Adalimumab: standard criteria; golimumab: standard criteria and disease duration; infliximab: standard criteria, BASDAI, and disease duration. ADA adalimumab, ASAS Assessment in Spondyloarthritis international Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, GOL golimumab, INF infliximab, SEC secukinumab, TNF tumor necrosis factor
Patients on secukinumab had higher ASAS 40 response versus those on adalimumab, golimumab, and infliximab at week 24 (Fig. 2). It was also higher versus adalimumab at week 52 (58% vs 47%).
3.2 Cost-per-Responder Analysis
-
CPR at week 24: The cost per ASAS 20 at week 24 for secukinumab was 75% lower than adalimumab, 65% lower than golimumab, and 80% lower than infliximab (Fig. 3). The cost per ASAS 40 at week 24 for secukinumab was 77% lower than adalimumab, 67% lower than golimumab, and 83% lower than infliximab (Fig. 3). Secukinumab dominated adalimumab, golimumab, and infliximab for both ASAS 20 and ASAS 40 responses at week 24, which means the costs were lower and efficacy was higher for secukinumab compared with the other biologics.
-
CPR at week 52: Cost per ASAS 20 at week 52 for secukinumab was 77% lower than adalimumab and 79% lower than infliximab (Fig. 4). The cost per ASAS 40 at week 52 for secukinumab was 79% lower than adalimumab (Fig. 4). Secukinumab dominated adalimumab for both ASAS 20 and ASAS 40 responses at week 52.
-
ICAR: Secukinumab dominated all comparators at week 24, which means the cost of secukinumab was lower and response was higher compared with the comparators.
-
NNT: Compared with adalimumab, the NNT to achieve ASAS 20 and ASAS 40 response was 7 at week 24 for secukinumab; at week 52 it was 12 and 9 respectively.
Cost per ASAS response at week 24. Standard matching criteria for all comparators: age, sex, C-reactive protein (CRP) levels, BASFI, and TNF-naive population. Adalimumab: standard criteria; golimumab: standard criteria and disease duration; infliximab: standard criteria, BASDAI, and disease duration. ADA adalimumab, ASAS Assessment in Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, GOL golimumab, IDR Indonesian rupiah, INF infliximab, SEC secukinumab, TNF tumor necrosis factor
Cost per ASAS response at week 52. Standard matching criteria for all comparators: age, sex, C-reactive protein (CRP) levels, BASFI, and TNF-naive population. Adalimumab: standard criteria; infliximab: standard criteria, BASDAI, and disease duration. ADA adalimumab, ASAS Assessment in Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, IDR Indonesian rupiah, INF infliximab, SEC secukinumab, TNF tumor necrosis factor
Compared with golimumab, the NNT to achieve both ASAS 20 response and ASAS 40 response at week 24 was 9 for secukinumab.
Compared with infliximab, the NNT to achieve ASAS 20 response and ASAS 40 response at week 24 was 6 and 5, respectively, for secukinumab.
-
TA Across all the comparators, the TA revealed that >65% reduction in efficacy of secukinumab or > 187% increase in secukinumab total cost was required to make secukinumab a non-cost-effective option for ASAS 20 response (Fig. 5). For ASAS 40 response, TA revealed that > 67% reduction in efficacy of secukinumab or > 202% increase in secukinumab total cost was required to make secukinumab a non-cost-effective option (Fig. 6).
4 Discussion
The Indonesian Rheumatology Association recommends NSAIDs, TNFi, and interleukin (IL)-17A inhibitor for the management of AS [2]. The clinical efficacy and safety of biologics in treating AS has been previously demonstrated [16,17,18]. Besides the clinical value, the economic value needs to be demonstrated. This study provides a comprehensive evaluation of the comparative cost effectiveness of approved systemic treatments in Indonesia for the treatment of AS by using Indonesian costs. This is the first attempt to conduct a CPR analysis among patients with AS in Indonesia.
Clinical efficacy (ASAS 20, ASAS 40) was found to be higher among patients on secukinumab versus those on infliximab, adalimumab, or golimumab at week 24 and at week 52.
Secukinumab dominated adalimumab, golimumab, and infliximab at week 24 and adalimumab at week 52 by being less costly and more efficacious. This finding is consistent with that of other studies from different countries (Table 3). All the studies considered only direct costs and derived the responder rates from MAIC except for a Korean study. The CPR demonstrated that using secukinumab will have higher clinical benefits at much lower cost [19,20,21,22,23].
Secukinumab also has a favorable safety profile over long-term treatment in patients with AS [16]. The common adverse event (AE) for all four comparators was upper respiratory tract infection (URTI). At week 102, URTI was lowest in patients on secukinumab (48.7% with infliximab, 21.1% with golimumab, 17% with adalimumab, and 10.4% with secukinumab) [24,25,26]. The estimated hospital cost for treating respiratory infection in Indonesia is IDR4,431,800 (INA-CBG code J-4-15-I, Regional 1, Government Hospital Type A) [27]. Lower URTI due to secukinumab will also reduce the cost burden on health facilities. Although not all TNFi and IL-17 inhibitor studies mentioned tuberculosis (TB) in the list of AEs, the burden of TB reactivation from latent TB infection should also be one of the considerations when choosing biologic treatment, especially in Indonesia where the incidence of tuberculosis is high [28, 29].
Real-life data from 13 European registries participating in the European Spondyloarthritis Research Collaboration demonstrated that patients on secukinumab show good adherence; secukinumab retention rates after 6 and 12 months of treatment were reported to be high [30].
The TA revealed that substantial reduction in efficacy of secukinumab or substantial increase in cost of secukinumab would make it not cost effective, indicating the robustness of the results.
4.1 Limitations
Despite being a lean economic valuation method, CPR has its limitations. It does not consider the utility benefits associated with differences in efficacy between comparators. In addition, it does not assess the long-term effects of comparators on cost and efficacy as it is limited to a maximum of 52 weeks. Moreover, the list price of the comparators was included, which does not include discounts offered for individual brands. The lack of real-world data from Indonesia is also a limitation of this study.
MAIC in this analysis (relative to adalimumab, golimumab, and infliximab) was conducted based on the pivotal trial data which didn’t consider adherence. Therefore, adherence couldn’t be considered in the analysis.
5 Conclusion
This study demonstrated that if AS patients in Indonesia were treated with secukinumab instead of comparator therapies, more patients could be treated, and more patients would reach response to treatment for the same budget.
Notes
Consensus from a survey (questionnaire) conducted by the Indonesian Rheumatologist Association sent to all Indonesian rheumatologists on biologic agent use in AS.
These drug-administration costs were calculated as the averages taken from a survey conducted in several centers that used biologic agents.
References
Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650–7.
Indonesia PR. Diagnosis dan pengelolaan spondiloartritis. Perhimpunan Reumatologi Indonesia; 2021 [Internet] https://reumatologi.or.id/rekomendasi-diagnosis-dan-pengelolaan-spondiloartritis-2021/. Accessed 10 July 2021.
Kim D, Kim H, Cho S, Park MC. A cost per responder analysis of secukinumab vs adalimumab for the treatment of ankylosing spondylitis from the Korean perspective. Int J Rheum Dis. 2019;22:1630–7.
Maksymowych WP, Strand V, Nash P, et al. Comparative effectiveness of secukinumab and adalimumab in ankylosing spondylitis as assessed by matching-adjusted indirect comparison. Eur J Rheumatol. 2018;5:216–23.
Maksymowych WP, Choy E, Yazici Y, Walsh JA, Thom H, Kalyvas C, Fox T, Gandhi K, Jugl S. Comparative Effectiveness of Secukinumab and Golimumab in Ankylosing Spondylitis: Assessed By Matching-Adjusted Indirect Comparison Using Pivotal Phase 3 Clinical Trial Data [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). [Internet] https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-and-golimumab-in-ankylosing-spondylitis-assessed-by-matching-adjusted-indirect-comparison-using-pivotal-phase-3-clinical-trial-data/. Accessed 5 June 2022.
Blair HA. Secukinumab: A review in ankylosing spondylitis. Drugs. 2019;79:433–43.
Phillippo DM AA, Dias S, Palmer S, Abrams KR, Welton NJ . Nice dsu technical support document 18: Methods for population-adjusted indirect comparisons in submission to nice, 2016 [Internet] https://research-information.bris.ac.uk/en/publications/nice-dsu-technical-support-document-18-methods-for-population-adj. Accessed 7 Apr 2021.
Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–7.
Thom H, Jugl S, Palaka E, Sunil J. Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals. Value Health. 2016;19:A100–A101. https://doi.org/10.1016/j.jval.2016.03.1723.
INDONESIA CSOTRO. Statistics Indonesia releases 2020 census results. 2021 [Internet]; https://setkab.go.id/en/statistics-indonesia-releases-2020-census-results/#:~:text=Statistics%20Indonesia%20(BPS)%20has%20released,141%20people%20per%20square%20kilometer.&text=Based%20on%20the%20distribution%20per,on%20the%20island%20of%20Java. Accessed 7 Apr 2021.
Baraliakos X, Kiltz U, Peters S, et al. Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiographic and non-radiographic axial spondyloarthritis. Rheumatology (Oxford). 2017;56:95–102.
AbbVie Biotechnology GmbH & Co KG. L, Germany. Fraizeron product information 2019; adalimumab summary of product characteristics. 2019 [Internet] http://pionas.pom.go.id/obat-baru/humira-larutan-injeksi-40-mg08-ml. Accessed 7 Apr 2021.
Novartis. Fraizeron® (secukinumab) 150 mg powder for solution for injection basic succinct statement (bss). 2020 [Internet] https://www.mims.com/indonesia/drug/info/fraizeron?type=full. Accessed 12 May 2021.
MIMS. Infliximab generic medicine info 2020 [Internet] https://www.mims.com/indonesia/drug/info/infliximab. Accessed 10 July 2021.
MIMS. Golimumab generic medicine info. 2021 [Internet] https://www.mims.com/indonesia/drug/info/golimumab?mtype=generic. Accessed 12 May 2021.
Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: Integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21:111.
Dubash S, Bridgewood C, McGonagle D, Marzo-Ortega H. The advent of il-17a blockade in ankylosing spondylitis: secukinumab, ixekizumab and beyond. Expert Rev Clin Immunol. 2019;15:123–34.
Liu W, Wu YH, Zhang L, et al. Efficacy and safety of tnf-α inhibitors for active ankylosing spondylitis patients: Multiple treatment comparisons in a network meta-analysis. Sci Rep. 2016;6:32768.
Patino A, Karpf E. Secukinumab as a more efficient alternative for the treatment of ankylosing spondylitis: a cost per responder analysis versus adalimumab and golimumab from a peruvian perspective for private and public health schemes. Value Health. 2017;20:A555.
Patino A, Karpf E. Secukinumab as a more efficient alternative for the treatment of ankylosing spondylitis: A cost per responder analysis versus adalimumab and golimumab from a colombian perspective. Value Health. 2017;20:A555.
Gunda P, Kandhare A, Nikoglou E, Jugl SM, Kneidl J, Neidhardt K. A cost per responder analysis of secukinumab vs adalimumab based on a matching-adjusted indirect comparison for the treatment of ankylosing spondylitis from a german payer perspective. Value Health. 2016;19:A537.
Walsh J, Nikoglou E, Praveen G, Park Y, Jugl S. Secukinumab Vs Adalimumab for the Treatment of Ankylosing Spondylitis: A Cost per Responder Analysis at 52 Weeks from the US Perspective [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). [Internet] https://acrabstracts.org/abstract/secukinumab-vs-adalimumab-for-the-treatment-of-ankylosing-spondylitis-a-cost-per-responder-analysis-at-52-weeks-from-the-us-perspective/. Accessed 5 June 2022.
Al Mudaiheem H, Al Howimel M, Al Jufan K, et al. Cost per-responder analysis of secukinumab compared to adalimumab for the treatmenmt of ankylosing spondylitis over one year in saudi arabia. Value Health. 2018;21:S81–2.
Van der Heijde D, Schiff MH, Sieper J, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the atlas trial. Ann Rheum Dis. 2009;68:922–9.
Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase iii study. Arthritis Care Res (Hoboken). 2017;69:1020–9.
Braun J, Deodhar A, Dijkmans B, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum. 2008;59:1270–8.
Kesehatan K. Peraturan menteri kesehatan tentang perubahan atas peraturan menteri kesehatan nomor 52 tahun 2016 tentang standar tarif pelayanan kesehatan dalam penyelenggaraan program jaminan kesehatan. 2016 [Internet]. https://peraturan.bpk.go.id/Home/Details/114477/permenkes-no-64-tahun-2016. Accessed 15 Mar 2022.
WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025 [Internet]. https://apps.who.int/iris/handle/10665/341980. Accessed 5 June 2022.
Evangelatos G, Koulouri V, Iliopoulos A, Fragoulis GE. Tuberculosis and targeted synthetic or biologic dmards, beyond tumor necrosis factor inhibitors. Ther Adv Musculoskelet Dis. 2020;12:1759720x20930116.
Michelsen B, Lindström U, Codreanu C, et al. Drug retention, inactive disease and response rates in 1860 patients with axial spondyloarthritis initiating secukinumab treatment: routine care data from 13 registries in the EuroSpA collaboration. RMD Open. 2020;6(3):e001280. https://doi.org/10.1136/rmdopen-2020-001280.
Acknowledgments
The authors thank G. S. Ramakrishna, Novartis Healthcare Private Limited, Hyderabad, India, for writing and editorial support. The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding Source
This study was sponsored by Novartis Indonesia.
Competing Interests
CS Wahono, L Hamijoyo, and H Isbagio were contracted by Novartis Indonesia for the study. Y Hendrawan and L Rakinaturia are employees of Novartis Indonesia. N Mittal, P Khanna, and M Jain are employees of Novartis Healthcare Private Limited, Hyderabad, India.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication (from patients/participants)
Not applicable.
Availability of Data and Material
All data generated or analysed during the study are included in this published article and its supplementary files.
Code Availability
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. Codes are embedded in the model.
Author Contribution Statement
All authors were involved in conducting the study. All authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wahono, C.S., Hamijoyo, L., Hendrawan, Y. et al. Secukinumab in Ankylosing Spondylitis Patients: A Cost-per-Responder Analysis from the Indonesian Health System Perspective. PharmacoEconomics Open 7, 605–615 (2023). https://doi.org/10.1007/s41669-023-00401-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00401-6