Abstract
Objective
This study assessed the cost-effectiveness of nivolumab plus ipilimumab versus both sunitinib and pazopanib for the treatment of first-line unresectable advanced renal cell carcinoma (aRCC) from a healthcare system perspective in Switzerland.
Methods
A three-state partitioned survival model, consisting of progression-free, progressed disease, and death, was constructed. Efficacy estimates were based on data from the CheckMate 214 trial (NCT02231749) with a minimum follow-up of 42 months. Two Swiss oncologists were consulted to determine disease management resource use. Costs were derived from the Swiss tariff lists for outpatient (TARMED Online Browser 1.09) and inpatient (2020 data from Swiss diagnosis-related groups) treatments. Drug acquisition costs (ex-factory prices) were obtained from the March 2020 price list published by the Swiss Federal Office of Public Health. Treatment-specific EQ-5D-3L-based utilities were derived from CheckMate 214 using a French value set as a proxy for Switzerland. The model utilized a 1-week cycle length and a 40-year time horizon, with costs and effects discounted by 3.0% per annum. One-way sensitivity analyses, probabilistic analysis, and scenario analyses assessed the robustness of the results.
Results
Nivolumab plus ipilimumab yielded incremental 1.43 life-years and 1.36 lifetime discounted quality-adjusted life-years (QALYs) relative to sunitinib and pazopanib at an additional cost of 147,453 Swiss Francs (CHF) and CHF145,643, respectively. With an incremental cost-utility ratio of CHF108,326 per QALY gained versus sunitinib, and CHF106,996 per QALY gained versus pazopanib, the nivolumab plus ipilimumab combination can be considered a cost-effective option for the treatment of patients with aRCC in Switzerland, with a willingness-to-pay threshold of CHF200,000. Sensitivity and scenario analyses confirmed the robustness of the deterministic results.
Conclusions
This study showed that nivolumab plus ipilimumab, which represents one of the standard-of-care first-line treatments for intermediate- or poor-risk aRCC patients, is a life-extending and cost-effective treatment option for patients in Switzerland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The incremental cost-utility ratio (ICUR) of nivolumab plus ipilimumab (NIVO + IPI) in the treatment of first-line renal cell carcinoma amounted to 108,326 Swiss Francs (CHF) per quality-adjusted life-year (QALY) gained versus sunitinib and CHF106,996 per QALY gained versus pazopanib. |
At a Swiss willingness-to-pay threshold of CHF200,000 per QALY, NIVO + IPI was considered cost effective. |
Sensitivity analyses showed the robustness of the deterministic model base-case outcomes. |
NIVO + IPI is a life-extending therapy for patients in Switzerland and a valuable addition to the treatment landscape. |
1 Introduction
Each year, 431,000 kidney cancer cases are diagnosed worldwide, resulting in 179,000 deaths [1]. Approximately 90% of all kidney malignancies can be labelled as renal cell carcinoma (RCC) [2]. Approximately 690 men and 310 women were diagnosed with RCC each year between 2013 and 2017 in Switzerland, where 196 deaths in men and 105 deaths in women were observed, equalling 2.01% and 1.4%, respectively, of all cancer deaths in Switzerland [3]. Alongside the aging population and rising prevalence of risk factors such as obesity, the incidence of advanced RCC (aRCC) is increasing worldwide. The burden of aRCC is therefore predicted to increase considerably [4, 5].
Twenty-one percent of patients with RCC are diagnosed at an advanced or metastatic stage, with tumour invasion through the fascia ligament covering the kidney, direct tumour extension to the adjacent adrenal gland, or spread to distant organ(s), such as the lung, liver, bone and brain [6, 7]. Furthermore, per International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria, 77% of patients diagnosed with intermediate- or poor-risk aRCC have worse outcomes than patients with favourable-risk disease [8, 9]. The classification of an intermediate- or poor-risk patient is based on the number of predefined risk factors present, as reported by Heng et al. [8, 9].
In Switzerland, over the past decade, most patients with aRCC received systemic first-line treatment with a tyrosine kinase inhibitor (TKI) such as sunitinib (SUN) or pazopanib (PAZ) [10]. Despite the achievements from introducing these standard-of-care TKIs for aRCC in the late 2000s, the most recent 5-year relative survival rate remains low at 13% [11]. However, disease prognosis has likely improved significantly after approval of combination immunotherapy treatments.
The combination of the PD-L1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab (NIVO + IPI) provides a relatively new treatment option for first-line aRCC in Europe. The efficacy of NIVO + IPI was assessed in CheckMate 214 (NCT02231749), a phase III randomized, controlled trial, and was first reported with 17.5-month minimum follow-up [12]. CheckMate 214 showed superior overall survival (OS; hazard ratio [HR] 0.63; p < 0.001) and objective response rate (ORR; 42% vs. 27%; p < 0.001) versus SUN. In 2020, updated data reported with 42-month minimum follow-up [13] confirmed the continued superior efficacy outcomes of NIVO + IPI versus SUN in intermediate- or poor-risk patients. Furthermore, a complete response (CR) rate of > 10% was observed with NIVO + IPI versus 1.4% for SUN, with 83.7% of CRs and 62.5% of partial responses to NIVO + IPI still ongoing with 42-month minimum follow-up [13]. Median duration of response was not reached in the NIVO + IPI arm versus 19.7 months in the SUN arm. These results, alongside results of the 5-year update published in 2022, confirmed the superiority of NIVO + IPI as a first-line treatment option with the potential for durable response [13, 14].
On 26 July 2018, Swissmedic approved NIVO + IPI for the treatment of patients with previously untreated intermediate- or poor-risk aRCC in Switzerland, joining other reimbursed treatments, i.e. PAZ, SUN, temsirolimus, and bevacizumab [15]. To our knowledge, no study in the literature compares cost and utility of NIVO + IPI versus the current reimbursed medical practice in Switzerland, which consists of SUN and PAZ in first line [10]. We present the cost-effectiveness of NIVO + IPI versus SUN or PAZ in these patients to better inform health technology assessment experts and policymakers regarding the long-term economic value of immune checkpoint inhibitors in first-line aRCC.
2 Methods
2.1 Population and Model Structure
The analyses were performed from a Swiss healthcare payer perspective, accounting for all direct medical costs to the healthcare system arising from patients, health insurers, and the Swiss cantons (administrative subdivisions). The population included intermediate- and poor-risk aRCC patients. Patient characteristics, such as median age and sex, were based on CheckMate 214 data [12]; average bodyweight, used to inform treatment dosing, was based on Swiss Federal Office of Public Health data [16].
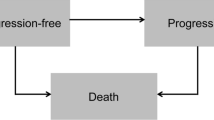
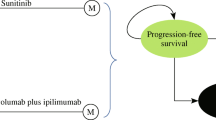
We developed a partitioned survival model using Microsoft Excel (Fig. 1) to evaluate the incremental cost-utility ratio (ICUR) and incremental cost-effectiveness ratio (ICER) of NIVO + IPI versus SUN or PAZ, which are first-line treatments for aRCC that are reimbursed in medical practices in Switzerland. The model comprised three health states (progression-free [PF], progressed disease [PD], and death) reflecting the natural history of aRCC.
The model used a 7-day cycle length, applying half-cycle correction. This time period was deemed sufficient to capture clinical outcomes from the CheckMate 214 trial and matched the dosing schedules of included treatments. Costs and effects were both discounted at 3% per annum, based on World Health Organization guidelines [17]. The model used a 40-year lifetime horizon, by which point < 1% of aRCC patients are predicted to be alive (Table 1), as sufficient to capture all costs and effects of the treatments [18].
2.2 Efficacy and Survival
Efficacy measures for NIVO + IPI versus SUN were based on the progression-free survival (PFS) and OS outcomes reported from CheckMate 214 at the August 2019 database lock (42-month minimum follow-up) [13]. The efficacy of PAZ, for which no head-to-head evidence is available from CheckMate 214, was considered clinically equivalent to that of SUN based on previous National Institute for Health and Care Excellence (NICE) health technology assessments [19,20,21,22,23]. Available clinical trial evidence includes demonstration of non-inferiority in the COMPARZ study, IMDC data showing similar real-world effectiveness, and similar recommendations in international guidelines (European Association of Urology, European Society for Medical Oncology, and National Comprehensive Cancer Network) [19,20,21,22,23].
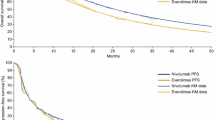
To extrapolate survival outcomes over a lifetime horizon, standard parametric and spline models were fitted to the Kaplan–Meier trial data for PFS—per independent radiologic review committee assessment, OS, and time to treatment discontinuation (TTD), which was used to inform treatment costs of all comparator treatments. NICE technical guidance [24] was followed for extrapolation and selection of the models.
The proportional hazards assumption was investigated using both qualitative assessments using log-cumulative hazard plots and Schoenfeld residuals visualization, as well as quantitative assessment using the Grambsch and Therneau test [25]. For both PFS and TTD, the proportional hazards assumption was violated, and independent distributions were fitted separately to survival data of both trial arms. For independent distributions, the same distribution was selected for both trial arms, per NICE technical guidance [24]. For the OS outcome, as the proportional hazards assumption could not be rejected, a single dependent model was fitted to both arms of the trial data.
Standard parametric distributions (exponential, Weibull, Gompertz, log-logistic, log-normal, gamma, and generalized gamma) and six cubic spline models (hazard spline, odds spline, and probit spline, each with one and two intermediate knots) were fitted to the CheckMate 214 data. Model fitting was assessed by means of statistical fit criteria including the Akaike information criterion (AIC) and Bayesian information criterion (BIC), clinical plausibility, visual inspection of survival curves, and hazard plots. PFS was compared against external sources for additional evidence in the SUN arm [26, 27].
For PFS, the independent one-knot odds spline provided the best fit and was used for both NIVO + IPI and SUN. For TTD, the independent log-logistic curve provided the best fit for both trial arms. A dependent log-normal distribution was chosen to model OS, in line with non-violation of the proportional hazards assumption, as it was the best-fitting model for both trial arms. Estimated hazard rates for OS were adjusted by Swiss background mortality rates to ensure that they do not exceed those of the general population [28]. Figure 1 gives an overview of the selected PFS and OS distributions for the NIVO + IPI, SUN, and PAZ arms as extrapolated in the model, accounting for background mortality. The figure shows that after a 10-year time period, PFS is adjusted by OS to make sure that PFS could not lie above OS. This implies that patients alive at 10 years are not expected to progress further. This can be confirmed by NICE clinician feedback, which stated that for patients alive at 10 years, the impact of disease was expected to be gone, and any survival events after this would be the same as those experienced by the general population [29]. Alternative survival models, including independent models for OS, were tested in sensitivity analyses (electronic supplementary material [ESM]). For SUN and PAZ, the PFS curve lies far below the OS, in which case a correction would not be needed. Furthermore, we estimate that applying the 10-year rule for SUN and PAZ would have limited impact on the results, as after 10 years, the currently used extrapolated PFS curve for these treatments also is shown to stabilize.
2.3 Costs and Resource Use
Two Swiss oncologists were consulted to retrieve or confirm various inputs that were used in the model (see the ESM). Dr. Vogl (Istituto Oncologico della Svizzera Italiana in Bellinzona) and Dr. Rothermundt (Kantonsspital St. Gallen) provided written inputs, which were discussed and consolidated during a virtual meeting.
2.3.1 Treatment Acquisition, Administration and Monitoring
The clinical experts consulted indicated that patients who achieved CR would receive at least 3 months of therapy thereafter in Switzerland. With a minimum of 42 months follow-up from CheckMate 214, 10.1% of NIVO + IPI and 1.4% of SUN patients had achieved CR [13]. Based on these rates and clinical expert opinion, an additional 3 months of treatment time was added to the median time to response as measured in the trial [13]. This led to a maximum treatment duration of 5.8 months for NIVO + IPI and 6.6 months for SUN among those who achieved CR. The maximum treatment duration for PAZ was assumed to be equal to SUN as we assume non-inferiority between treatments. As this assumption comes with uncertainty, a scenario analysis extending the treatment period to 24 months after CR was conducted based on recommendations from the experts.
With 42-month minimum follow-up, subsequent systemic therapy rates among patients who experienced disease progression were 66% and 71% for NIVO + IPI and SUN, respectively [13]. CheckMate 214 data (base case) and clinical expert opinion (scenario analysis) guided subsequent treatment distributions. Mean subsequent treatment duration was derived from the publication by Sandmeier et al. [10]. ESM Table S4 provides the proportions of patients receiving each of these subsequent treatments, according to the first-line therapy received.
Treatment acquisition, administration and monitoring costs were calculated using the TTD curves of each arm, considering the maximum treatment duration for patients with CR. This was incorporated in the models as CheckMate 214 patients who discontinued therapy due to either disease progression on treatment or severe toxicity caused by the treatment without progression. This is also seen in CheckMate 214, where at 42 months, 13% of NIVO + IPI and 7% of SUN patients remained on their original therapy, whereas 31% of NIVO + IPI and 12% of SUN patients were surviving free of subsequent therapy [30]. PAZ TTD was assumed to be equal to SUN TTD, as the CheckMate 214 trial did not include PAZ. Drug acquisition costs were based on ex-factory drug prices published by the Swiss Federal Office of Public Health (positive drug list, March 2020) [31]. Unit costs for drug administration and disease management were derived from TARMED Browser 1.09 [32] and the Bundesamt für Gesundheit quick analysis list 2020 [33]. Costs were indexed to 2020 prices where needed.
The Swiss oncologists provided input regarding resource use required to manage disease, including outpatient visits, monitoring and scans, and terminal care (ESM Table S5). Grade 3/4 treatment-related adverse events (AEs) due to any cause with an incidence ≥ 2% in CheckMate 214 (NIVO + IPI and SUN) or COMPARZ (PAZ) for either arm were included. Only hospitalization costs were taken into consideration for the AEs, which were derived from 2020 Swiss diagnosis-related group data (DRG 2020). Based on clinical expert opinion, 20% of patients with a grade 3 or 4 AE get hospitalized in Switzerland and therefore incur the costs listed in DRG 2020. Finally, inpatient terminal care was included for 30% of aRCC patients in both trial arms in the last cycle before their death, based on clinical expert opinion, with costs derived from DRG 2020 [34].
2.4 Utilities
Health-related quality-of-life data were collected during the CheckMate 214 trial using EQ-5D-3L [35]. In the absence of a Swiss-specific value set, French population-based preference weights were applied to the data [36]. A statistically significant difference in pre-progression utility was observed between treatment arms; therefore, treatment-specific health state utility values for NIVO + IPI and SUN were assigned to each corresponding arm: 0.824 (standard error [SE] 0.003) for NIVO + IPI and 0.769 (SE 0.004) for SUN and PAZ. After progression, non-treatment-specific utilities (0.772, SE 0.005) were applied to each arm, irrespective of first-line therapy received. For PAZ, the model assumes the same pre-progression utility as the SUN arm. Scenario analyses included setting the same utility for both treatment arms and using a German value set instead of a French value set [37]. As treatment-specific utilities were used in PFS, disutilities for AEs were not separately accounted for in the model, as this could lead to double counting.
2.5 Sensitivity Analyses
A one-way sensitivity analysis (OWSA) and a probabilistic analysis were performed to assess whether the base-case results were robust against parameter uncertainty. For each parameter considered in OWSA, ranges for the OWSA were determined according to the observed 95% confidence interval (CI) or SE of the corresponding parameter value. When such values were missing, as a proxy, upper and lower extremes were used by varying the mean value of the corresponding parameter 10% up and down. Parameters tested in both analyses included patient bodyweight, utility estimates for PF and PD states, efficacy estimates based on the 95% CI, the percentage of patients receiving subsequent treatment, AE incidence, and resource use and their respective unit costs. The probabilistic analysis was performed with 1000 Monte Carlo iterations, which was deemed sufficient for stabilization. Scenario analyses assessed model robustness (ESM Table S6).
3 Results
3.1 Deterministic Outcomes
Over a 40-year lifetime horizon, mean discounted survival for patients treated with NIVO + IPI was estimated to be 6.00 years, with 25% of patients surviving at least 10 years. Mean discounted survival for patients treated with SUN or PAZ was estimated at 4.57 years, with 17% of patients surviving at least 10 years. This resulted in an incremental lifetime gain for NIVO + IPI of 1.43 life-years (LYs) versus SUN or PAZ. Total lifetime discounted quality-adjusted life-years (QALYs) for patients receiving NIVO + IPI were estimated as 4.88 QALYS versus 3.52 QALYS for SUN or PAZ—an incremental gain of 1.36 QALYs in favour of NIVO + IPI.
NIVO + IPI treatment was associated with a total cost of CHF284,007 over a lifetime horizon, versus CHF136,554 for SUN and CHF138,365 for PAZ (Table 2). Although total treatment costs were higher for NIVO + IPI, cost savings were observed versus both SUN and PAZ in terms of disease management in the PD state, subsequent treatment, AEs, and terminal care. SUN was associated with slightly lower drug acquisition and PF disease management costs versus PAZ but had higher AE management costs due to differences in toxicity profiles.
The cost-effectiveness efficiency frontier is formed by SUN and NIVO + IPI (Fig. 2), while PAZ is dominated due to slightly higher total costs than SUN. NIVO + IPI treatment is associated with a cost increase of CHF147,453 relative to SUN, and CHF145,643 relative to PAZ, and an additional 1.36 QALYs versus both therapies. Therefore, based on pairwise comparisons, the discounted ICUR for NIVO + IPI versus SUN and PAZ is CHF108,326/QALY and CHF106,996/QALY, respectively.
3.2 Sensitivity Analyses
NIVO + IPI had a 97% probability of being cost effective at a willingness-to-pay (WTP) threshold of CHF200,000/QALY (Fig. 3) [38]. When evaluating all three treatments, NIVO + IPI had the highest probability of being cost effective at a WTP threshold of CHF106,250/QALY. Below this WTP threshold, SUN had the highest probability of being cost effective, while PAZ never exceeded 17% probability of being cost effective at any WTP threshold. With an assumption of equal efficacy between SUN and PAZ, slightly higher costs of PAZ versus SUN drives the differences in the cost-effectiveness outcomes.
Scenario analyses were performed to measure the impact of the base-case structural modelling choices over the main results of the analysis (ESM Table S6). The analyses show that most assumptions made in the base case seem to be robust, with the selection of an alternative OS distribution (dependent hazard spline one-knot or independent log-normal) leading to the greatest deviance from the base case, with a decrease of 22.2% and 27.2% in the ICUR versus SUN and PAZ, respectively. The ICUR was increased by 16.6% when switching from a weight-based dosing (per label) to a flat dose regimen, or by 16.5% when assuming non-treatment-specific utilities in the PFS state. The other scenarios resulting in an increased ICUR were the assumption of a maximum treatment duration of 24 months after CR (+ 3.8%) and using an independent generalized gamma distribution for PFS, which was the second-best performing distribution (+ 2.0%). All other scenarios resulted in a reduction of the ICUR ranging from − 2.3 to − 8.2%.
The OWSA results showed that the proportion of patients with progressive disease who received subsequent treatment, and the mean PFS in the second-line setting, were the main drivers of uncertainty in the model. However, both parameters had limited impact, as the lower and upper bounds of the ICUR remained between CHF100,000/QALY and CHF117,000/QALY.
4 Discussion
This study demonstrated the cost-effectiveness of dual combination immunotherapy with NIVO + IPI versus the TKI monotherapies SUN and PAZ in previously untreated intermediate- or poor-risk aRCC patients in Switzerland. In the base-case analysis, NIVO + IPI yielded 1.43 incremental LYs and 1.36 incremental QALYs relative to SUN and PAZ at an additional cost of CHF147,453 and CHF145,643, respectively, over a 40-year lifetime horizon. Treatment with NIVO + IPI resulted in cost savings in terms of disease management in the PD state, subsequent treatment, AEs, and terminal care. The ensuing ICUR was CHF108,326 per QALY gained versus SUN, and CHF138,365 per QALY gained versus PAZ, which can be considered cost effective at a WTP threshold of CHF200,000 per QALY [38].
Sensitivity analyses demonstrated that model outcomes are robust. Scenario analyses indicated that most of the base-case assumptions were considered conservative. In particular, adopting an alternative assumption for OS had a significant impact, as the ICUR was shown to drop between 22.2% and 27.2%. The proportion of patients with progressive disease who received subsequent treatment, and the mean PFS in the second-line setting, were the main drivers of uncertainty in the model, as shown in the OWSA. However, both parameters had limited impact, as the lower and upper bounds of the ICUR remained between CHF100,000/QALY and CHF117,000/QALY in the OWSA. Finally, the probabilistic analysis showed that NIVO + IPI had a 97% chance of being the most cost-effective treatment at a WTP threshold of CHF200,000/QALY.
In 2019, the cost-effectiveness of NIVO + IPI in Switzerland was assessed based on an 18-month database lock [39]. In this analysis, NIVO + IPI was associated with a higher total number of QALYs versus SUN (increment of 1.27 QALYs) at an increased total cost (increment of CHF97,720), with a corresponding ICUR of CHF77,313/QALY versus SUN. Our study provides an updated cost-effectiveness analysis using a more recent 42-month database lock, with the addition of PAZ as a second comparator. Compared with the previous analysis, the new database lock resulted in a higher incremental QALY gain for NIVO + IPI due to more accurate long-term PFS and OS estimates [39]. However, estimated costs were also substantially higher, which was driven mainly by the application of a more conservative maximum treatment duration. In the previous analysis, all patients were assumed to stop treatment after 24 months, whereas in this analysis, an alternative rule was explored where only patients with a CR stopped treatment 3 months post-CR [39]. This assumption was informed by the literature [13] as well as Swiss medical expert input. We would like to point out that the NIVO maximum treatment duration rule (3 months after achieving CR) may be debated by other RCC experts and executed differently in clinical routine practice. Therefore, this assumption was tested in a scenario analysis to assess its effects on the ICUR, resulting in limited impact (3.8% increase).
The cost-effectiveness of NIVO + IPI versus SUN in aRCC has been assessed from a payer perspective in other countries. The study by Wu et al., based on the initially published 18-month database lock, resulted in ICURs varying from $85,506/QALY in the US, $126,499/QALY in the UK, and $4682/QALY in China, versus SUN [40]. In their analysis, they employed an independent Weibull and log-logistic distribution, resulting in an incremental QALY gain between 0.70 and 0.76. The discrepancy with our results is most likely due to the difference in time horizon, which was 10 years in the study by Wu et al., whereas we assumed a 40-year lifetime horizon.
In the study by Wan et al. [41], after data visualization of the published curves and deriving pseudo-individual patient-level data, AIC deemed the log-logistic distribution most fitting for PFS, while a Weibull distribution was chosen for OS. This resulted in an incremental 1.27 LY and 0.96 QALY gain, respectively, at a cost of $108,363 per QALY. This can be considered lower versus the 1.43 LY and 1.36 QALY estimates from our analysis. The Weibull distribution was also applied by Reinhorn et al., resulting in a US ICUR of $125,739/QALY over a 10-year time horizon [42]. It is important to note that Wan et al. and Reinhorn et al. made use of the primary database lock, with a minimum follow-up of 18 months. Survival analyses using more recent database locks, varying from a minimum follow-up of 30–48 months, showed that the dependent log-normal distribution is the best-performing distribution across all database locks for OS. Furthermore, although NIVO + IPI results remain robust across database locks, due to emerging signs of an OS plateau, the long-term estimates of the primary database lock of 18 months seem to be underestimated versus the newer database locks [43, 44]. This resulted in conservative incremental LYs and QALYs gained, as well as more conservative ICURs when using the 18-month database lock.
Some limitations exist with this analysis. First, it was not possible to validate long-term OS estimates for SUN or PAZ, as based on CheckMate 214 data, with external data, in particular with real-world outcomes from Swiss aRCC patients. Recent advancements in subsequent therapies, such as NIVO monotherapy, cabozantinib monotherapy, or lenvatinib plus everolimus, offering higher survival versus prior therapies, have not yet been captured in published observational studies [45,46,47]. A systematic literature review did not identify any effectiveness evidence for SUN or PAZ conducted after the introduction of these therapies [48]. Using older studies to validate OS extrapolations would likely lead to lower estimates that may not fully reflect today’s treatment landscape, and therefore local clinical experts were consulted. Furthermore, the efficacy of PAZ was assumed to be equal to that of SUN, as no direct evidence for PAZ was available from the CheckMate 214 trial. Although several publications suggest the clinical non-inferiority of SUN versus PAZ [19,20,21,22,23], an alternative strategy of implementing indirect comparison-based evidence may have led to slightly different results in the model.
Another limitation is the uncertainty around the utilities. As no Swiss-specific EQ-5D-3L value set was available at the time of this writing, a French value set was used in the base case. The impact of using an alternative German value set was assessed in a scenario analysis, showing limited impact on the results (ESM Table S6). A third limitation concerns the proportion of patients who receive subsequent systemic therapy and the distribution of therapies used in Swiss clinical practice versus subsequent therapies received in CheckMate 214. In the base case, it is assumed that the percentage of patients receiving subsequent therapy after treatment with PAZ was the same as the SUN arm from CheckMate 214. In the absence of other available data, this assumption was made on the basis that patients who had not yet received NIVO as part of their first-line therapy would then be eligible to receive it in second line. The distribution of treatments received would therefore be similar to that of the SUN arm in the CheckMate 214 trial. It is considered likely that the percentage of patients receiving any form of subsequent systemic therapy is higher in Switzerland versus the percentage provided in CheckMate 214 (66% of patients post-treatment with NIVO + IPI); however, exact numbers are unavailable. We sought clinical expert opinion to reflect this from a cost perspective in a scenario analysis, which showed moderate impact on the ICUR. However, it was not possible to properly adjust the efficacy data when using these alternative subsequent treatment estimates.
Finally, this analysis only included comparators that were assumed clinically relevant and were licensed and reimbursed in Switzerland, resulting in the inclusion of SUN and PAZ. However, the treatment field of aRCC is evolving rapidly and new drug combinations have already been licensed in Switzerland, namely the combination of pembrolizumab plus axitinib, pembrolizumab plus lenvatinib, and NIVO plus cabozantinib, which are not yet reimbursed. Future discussions regarding the most efficacious and cost-effective treatments for patients with aRCC warrant consideration of the CLEAR and KEYNOTE-426 trial data [49, 50]. Furthermore, it would be interesting to assess how the introduction of NIVO + IPI would impact the cost-effectiveness of NIVO in second-line aRCC, which has shown to be cost effective in previous analyses [51, 52]. Another interesting scenario to explore includes conducting a cost-effectiveness analysis from a societal perspective, so that the full societal impact of introducing NIVO + IPI in first line could be assessed.
5 Conclusion
This analysis has demonstrated that NIVO + IPI is an important cost-effective treatment option for patients with previously untreated intermediate- or poor-risk aRCC in Switzerland. NIVO + IPI is associated with a substantially longer incremental life expectancy (1.43 LYs) versus SUN and PAZ. Furthermore, numerically fewer high-grade AEs and a better health-related quality of life were reported with NIVO + IPI versus SUN for intermediate- and poor-risk aRCC patients [13]. The sensitivity analyses confirmed the robustness of the deterministic results, showing that NIVO + IPI provides a cost-effective treatment option, as already recommended in the current guidelines.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–24.
Federal Statistical Office. Neuerkrankungen und Sterbefälle nach Kreblokalisation 2013-2017. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/krankheiten/krebs/spezifische.assetdetail.14816151.html. Accessed 15 June 2021.
Gupta K, Miller JD, Li JZ, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205.
Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14(5):288–301.
National Cancer Intelligence Network (NCIN). Kidney cancer: survival report (urological cancers SSCRG). NCIN; 2014.
Cancer Research UK (CRUK). Kidney cancer statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer#heading-Zero. Accessed 15 June 2020.
Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8.
Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9.
Sandmeier N, Rothschild SI, Rothermundt C, et al. Pattern of care study in metastatic renal-cell carcinoma in the preimmunotherapy era in Switzerland. Clin Genitourin Cancer. 2018;16(4):E711–8.
National Cancer Institute, Howlader N, Noone AM, et al. 2019. SEER cancer statistics review, 1975–2016, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. https://seer.cancer.gov/archive/csr/1975_2016/. Accessed 15 June 2021.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8(2):e000891. https://doi.org/10.1136/jitc-2020-000891.
Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085–97.
Swissmedic. Swissmedic Journal 01/2018. 16. Jahrgang.
Bundesamt für Statistik. Schweizerische Gesundheitsbefragung 2012. https://www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/publikationen.assetdetail.349056.html. Accessed 10 June 2021.
World Health Organization. WHO guide to cost-effectiveness analysis. http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 3 Mar 2021.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10–31.
Powles T, Albiges L, Staehler M, et al. Updated European Association of Urology guidelines: recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2018;73(3):311–5.
Powles T, Albiges L, Bex A, et al. ESMO clinical practice guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol. 2021;32(15):1511–9.
National Institute for Health and Care Excellence (NICE). Cabozantinib for untreated advanced renal cell carcinoma—technology appraisal guidance (TA542). NICE; 2018.
Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31.
Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66.
Latimer N. NICE DSU Technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. NICE; 2011.
Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26.
Bozkurt O, Hacibekiroglu I, Kaplan MA, et al. Is late recurrence a predictive clinical marker for better sunitinib response in metastatic renal cell carcinoma patients? Clin Genitourin Cancer. 2015;13(6):548–54.
Kubackova K, Melichar B, Bortlicek Z, et al. Comparison of two prognostic models in patients with metastatic renal cancer treated with sunitinib: a retrospective, registry-based study. Target Oncol. 2015;10(4):557–63.
Bundesamt für Statistik. Vollständige jährliche Sterbetafel (Frauen), 2019. Vollständige jährliche Sterbetafel (Männer), 2019. https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/geburten-todesfaelle/lebenserwartung.assetdetail.14387086.html and https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung.assetdetail.14387068.html. Accessed 10 July 2021.
National Institute for Health and Care Excellence. Single technology appraisal. Nivolumab with ipilimumab for untreated metastatic renal cell carcinoma (CDF review of TA581) [ID3880]. Comittee papers. https://www.nice.org.uk/guidance/ta780/documents/committee-papers. Accessed 10 Jan 2022.
Regan M, Jegede OA, Mantia C, et al. Treatment-free survival, with and without toxicity, after immuno-oncology vs targeted therapy for advanced renal cell carcinoma (aRCC): 42-month results of CheckMate 214. Abstract 713P. Ann Oncol. 2020;31(Suppl 4):S561.
Swiss Federal Office of Public Health. Spezialitätenliste 2020.
TARMED Online Browser 1.09. https://www.tarmed-browser.ch/de. Accessed 31 Mar 2020.
Bundesamt für Gesundheit. Quick analysis list 2020. https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Analysenliste.html. Accessed 15 Mar 2021.
Swiss DRG. Fallkostenstatistik 2020. https://www.swissdrg.org/de. Accessed 12 Mar 2021.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14(1):57–66.
Greiner W, Claes C, Busschbach JJV, et al. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005;6(2):124–30.
Beck K, von Wyl V, Telser H, et al. Kosten und Nutzen von medizinischen Behandlungen am Lebensende, Nationales Forschungsprogramm NFP 67; 2016.
Oniangue-Ndza C, Schneider R, Malcolm B, et al. Cost-effectiveness of nivolumab in combination with ipilimumab compared to sunitinib for the first-line treatment of advanced/metastatic intermediate- or poor-risk renal cell carcinoma in Switzerland. Abstract PCN140. Value Health. 2019;22(Suppl 3):S462.
Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. 2018;6(1):124.
Wan X, Zhang Y, Tan C, et al. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5(4):491–6.
Reinhorn D, Sarfaty M, Leshno M, et al. A cost-effectiveness analysis of nivolumab and ipilimumab versus sunitinib in first-line intermediate- to poor-risk advanced renal cell carcinoma. Oncologist. 2019;24(3):366–71.
Çakar E, May J, Malcolm B, et al. Stability of lifetime overall survival estimates of nivolumab+ipilimumab in first-line advanced/metastatic intermediate- or poor-risk renal cell carcinoma. Abstract PCN421. Value Health. 2019;22(Suppl 3):S517–8.
Qendri V, May J, Malcolm B, et al. Updated lifetime survival estimates for nivolumab + ipilimumab in previously untreated advanced/metastatic intermediate- or poor-risk renal cell carcinoma (1L aRCC). Abstract PDG5. Value Health. 2020;23(Suppl 3):S521.
European Medicines Agency. Opdivo: EPAR—product information. https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf. Accessed 11 Nov 2021.
European Medicines Agency. Cabometyx: EPAR—product information. https://www.ema.europa.eu/en/documents/product-information/cabometyx-epar-product-information_en-0.pdf. Accessed 10 Nov 2021.
European Medicines Agency. Kisplyx: EPAR—product information. https://www.ema.europa.eu/en/documents/product-information/kisplyx-epar-product-information_en.pdf. Accessed 10 Nov 2021.
Trip AM, May J, Malcolm B, et al. Real-world effectiveness in previously untreated, advanced/metastatic renal cell carcinoma—a systematic literature review update. Abstract PCN42. Value Health. 2020;23(Suppl 2):S428.
Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300.
Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73.
Giuliani J, Bonetti A. Nivolumab is a cost-effective second-line treatment for metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2018;16(3):e557–62.
Giuliani J, Bonetti A. Cost-effectiveness of second-line treatments for metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2019;17(2):e258–62.
Acknowledgements
The authors would like to acknowledge Bristol Myers Squibb (BMS; Princeton, NJ, USA) and Ono Pharmaceutical Company Ltd (Osaka, Japan). All authors contributed to and approved the manuscript; Murat Kurt and Matthew Dyer (BMS) contributed to the writing of this article. Editorial assistance was provided by Parexel, funded by BMS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by BMS.
Conflicts of interest
Esra Çakar reports funding (institutional) from BMS to write this manuscript. Ursula M. Vogl reports honoraria (institutional) from Merck, BMS, Merck Sharp & Dohme (MSD), Pfizer, Janssen, Astellas, Roche, Bayer, Ipsen, and Sanofi, and (personal) from the Swiss Academy of Multidisciplinary Oncology (SAMO), Inselspital Bern, and Healthbook; payment for expert testimony (institutional) from Merck; travel support from Merck and Ipsen; and advisory board fees from Merck, BMS, MSD, Pfizer, Janssen, Astellas, Roche, Bayer, Ipsen, and Sanofi. Christian Rothermundt reports consulting fees from Merck (Schweiz) AG (personal) and Pfizer, BMS, MSD Oncology, Bayer (Schweiz) AG, and Ipsen (institutional). Sven L. Klijn reports consulting fees (institutional) from Bayer, BMS, Kite, and Janssen. César Oniangue-ndza, Ralph P. Schneider, and Jessica R. May are employed by and have stock ownership in BMS.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
BMS’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Code availability
Not available. Code is intellectual property of OPEN Health.
Authors’ contributions
Conception and design: CO, RPS, SLK and JRM. Data acquisition: EÇ, UMV and CR. Data analysis: EÇ and SLK. Data interpretation: EÇ, CO, RPS, SLK, UMV, CR and JRM. Writing of the manuscript: EÇ, CO, RPS, SLK, UMV, CR and JMR.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Çakar, E., Oniangue-Ndza, C., Schneider, R.P. et al. Cost-Effectiveness of Nivolumab Plus Ipilimumab for the First-Line Treatment of Intermediate/Poor-Risk Advanced and/or Metastatic Renal Cell Carcinoma in Switzerland. PharmacoEconomics Open 7, 567–577 (2023). https://doi.org/10.1007/s41669-023-00395-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00395-1