Abstract
Objective
The aim of this study was to evaluate the cost-utility of nivolumab plus ipilimumab (NIVO + IPI) versus other first-line therapies for advanced melanoma in the United States (US) from the third-party payer perspective.
Methods
This analysis estimated total expected life-years (LYs), quality-adjusted LYs (QALYs), and costs for first-line treatments of advanced melanoma during a 30-year time horizon using indirect treatment comparisons based on time-varying hazard ratios (HRs) and a three-state partitioned survival model. Overall survival (OS) and progression-free survival reference curves were extrapolated based on 5-year follow-up from the phase III Checkmate 067 trial (NCT01844505). Comparators of NIVO + IPI were NIVO, IPI, pembrolizumab, dabrafenib plus trametinib, encorafenib plus binimetinib (ENCO + BINI), and vemurafenib plus cobimetinib. Drug acquisition costs, treatment administration costs, follow-up time, subsequent therapy data, and adverse event frequencies were obtained from published sources. Utility weights were estimated from Checkmate 067, which compared NIVO + IPI or NIVO monotherapy with IPI monotherapy as first-line therapy in advanced melanoma. A 3% annual discount rate was applied to costs and outcomes. Sensitivity scenarios for BRAF-mutant subgroups were conducted.
Results
NIVO + IPI was estimated to generate the longest OS and the highest total costs versus all comparators, accruing 6.99 LYs, 5.70 QALYs, and $469,469 over the 30-year time horizon. The incremental cost utility of NIVO + IPI versus comparators ranged from $2130 per QALY (versus ENCO + BINI) to $76,169 per QALY (versus NIVO). In all base-case and most sensitivity analyses, the incremental cost-utility ratios for NIVO + IPI were below $100,000 per QALY.
Conclusions
NIVO + IPI is estimated to be a life-extending and cost-effective treatment versus other therapies in the US, with base-case incremental cost-utility ratios below $100,000 per QALY.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recommended first-line treatments for advanced melanoma include nivolumab plus ipilimumab (NIVO + IPI) and BRAF + MEK inhibitor (BRAFi + MEKi) combinations (for BRAF-mutant disease). |
At the time of this analysis, there was no head-to-head comparison of NIVO + IPI and BRAFi + MEKi combinations reported from a clinical trial setting in the literature, although indirect treatment comparisons have shown a clear survival benefit associated with NIVO + IPI versus BRAFi + MEKi combinations. |
The results of this analysis highlight the additional clinical and economic value of adding IPI to NIVO for first-line treatment of advanced melanoma. |
1 Introduction
Melanoma is a serious form of skin cancer. It is estimated that more than 100,000 new cases of melanoma will be diagnosed in the United States (US) in 2021 [1]. Patients with advanced disease have a poor prognosis. Based on registry data, approximately 4% of patients diagnosed with melanoma between 2010 and 2016 had distant metastases, and these patients demonstrated a 5-year survival rate of 27% [1]; however, this 5-year survival rate represents a marked improvement from that observed between 2003 and 2009 (16%) [2].
Treatments for advanced unresectable melanoma have changed dramatically over the last decade with the approval of non-chemotherapy systemic treatments, including immune checkpoint inhibitors (i.e., immuno-oncology [I-O] therapies) and targeted therapies (BRAF and/or MEK inhibitors) [3]. US treatment guidelines recommend the use of the I-O therapies nivolumab (NIVO), pembrolizumab (PEMBRO), or the combination of NIVO plus ipilimumab (NIVO + IPI) regardless of BRAF mutation status as first-line treatment, or the combination of BRAF and MEK inhibitors (dabrafenib plus trametinib [DAB + TRAM], vemurafenib plus cobimetinib [VEM + COBI], encorafenib plus binimetinib [ENCO + BINI]) if the patient has BRAF-mutant disease [4].
Although randomized controlled trials (RCTs) have reported overall survival (OS) data with 4 or 6.5 years of follow-up for each of these regimens, there were no head-to-head comparisons of I-O therapies and BRAF inhibitors or BRAF + MEK inhibitor combinations reported in the literature at the time of this analysis. RCTs of NIVO, NIVO + IPI, or PEMBRO have included comparisons with IPI monotherapy (CheckMate 067 [5,6,7], CheckMate 069 [8], and KEYNOTE-006 [9, 10]) or dacarbazine (CheckMate 066 [11]), whereas RCTs with BRAF + MEK inhibitors (coBRIM [12, 13], COMBI-d [14, 15], COMBI-v [15,16,17], and COLUMBUS [18]) only included comparisons with BRAF inhibitor monotherapy and were restricted to patients with BRAF-mutant melanoma.
I-O therapies have demonstrated a sustained plateau of long-term OS in a subset of patients regardless of BRAF status [6, 19]. BRAF + MEK inhibitor combinations have also shown the potential for a plateau of long-term OS, although not necessarily for similar proportions of patients with BRAF-mutant disease, as has NIVO + IPI (5-year OS rates, 34% with DAB + TRAM and 60% with NIVO + IPI) [6, 15]. Previous indirect treatment comparisons (ITCs) have demonstrated that these differences in long-term OS result in nonproportional hazards (i.e., hazard ratios [HRs] that vary over time) [20, 21]. A recent systematic literature review (SLR) of economic evaluations in advanced melanoma showed that only one study examined all licensed treatments (from a Norwegian perspective), with that study based on an ITC that assumed constant HRs [22]. Earlier economic evaluations of NIVO + IPI (from a US perspective) found this regimen to be cost ineffective, but these analyses were based on early progression-free survival (PFS) data only (prior to disclosure of OS data from CheckMate 067) and had a limited list of comparators [23, 24]. Consequently, there is a gap in the literature for a comprehensive economic evaluation of all recommended therapies that is based on robust ITCs accounting for differences in long-term OS by drug class. This analysis evaluated the cost-utility of NIVO + IPI in the first-line treatment of advanced melanoma using extrapolations of 5-year OS data from CheckMate 067 [6], which were the most current data available at the time of the analysis from a third-party US-payer perspective.
2 Methods
2.1 Model Overview
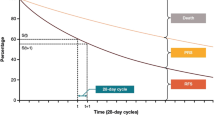
This cost-utility analysis was adapted from earlier versions of a model developed in support of health technology assessments for NIVO + IPI [25,26,27]. The analysis was conducted in a three-state framework (pre-progression, post-progression, and death) via a partitioned survival analysis. In this framework, the occupancy of each health state was calculated based on parametric PFS and OS curves. The cumulative survival probabilities for PFS and OS were used to estimate the number of patients occupying each health state in each cycle using the following equations:
-
Pre-progression = P(PFS)
-
Post-progression = P(OS) − P(PFS)
-
Death = 1 − P(OS)
The total cost and effectiveness of each treatment was calculated by combining the estimated time spent in the pre-progression and post-progression states with the costs and health utilities assigned to those states. The model assigned drug, disease management, and adverse event (AE) costs using monthly cycles, with half-cycle correction, over a 30-year time horizon (approximating a lifetime time horizon for a population with a starting age of 60 years [6]). Health state-specific utility weights were used to adjust for quality of life, and the quality-of-life impacts of AEs were also included. Health outcomes included accrued life-years (LYs) and quality-adjusted LYs (QALYs). This analysis was conducted from the US payer perspective and only included direct medical costs. All costs and outcomes were discounted using an annual 3% discount rate [28].
Comparators to NIVO + IPI in the model were comprised of NIVO, PEMBRO, DAB + TRAM, VEM + COBI, ENCO + BINI, and IPI. Incremental analysis is presented summarizing the differences in costs and health benefits between the competing treatments as incremental cost-utility ratios (ICURs; cost per QALY) for NIVO + IPI. The model was implemented in Microsoft Excel using Visual Basic for Applications (Microsoft Corporation, Redmond, WA, USA). All statistical analyses of trial data were performed using Stata 15 (StataCorp LLC, College Station, TX, USA) or SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
2.2 Clinical Data
Clinical data for NIVO + IPI were based on the phase III CheckMate 067 trial (NCT01844505), which compared NIVO + IPI or NIVO monotherapy with IPI monotherapy as first-line treatment of patients with unresectable/metastatic, stage III/IV melanoma [5, 6]. Comparator evidence was sourced from an SLR of RCTs that was completed in January 2020 (updated from a previously published SLR [20]). Treatments were evaluated in patients with previously untreated unresectable stage III/IV melanoma. A summary of the studies identified in the SLR and included in the network of evidence for ITCs is provided in the Electronic Supplementary Appendix (Section 1).
2.3 Survival Estimation
This analysis leveraged ITCs based on time-varying HRs. In the primary analysis, survival curves for each treatment were based on a network meta-analysis (NMA) of OS and PFS outcomes using individual patient data from CheckMate 067 (60-month database lock) [6] and published evidence identified in the SLR (Fig. 1a, b). The NMA was conducted as a fractional polynomial analysis, allowing determination of time-varying HRs. Based on observed survival trends and because both OS and PFS HRs significantly changed over time during the follow-up of each trial, an NMA that could capture these variations was considered to be statistically and visually more appropriate than a proportional hazards NMA that assumed and produced constant HR estimates for relative treatment effects. The best-fitting models were selected based on deviance information criteria, visual inspection, and model parsimony. IPI monotherapy was the reference treatment for all comparisons. Although relative treatment effects for OS and PFS were evaluated in networks assuming time-varying HRs, the HRs estimated at the end of the trial follow-up were carried onward as constants beyond the observed data.
Survival estimations used in the cost-utility analysis included the following: a OS, primary analysis, NMA all-comers; b PFS, primary analysis, NMA all-comers; (c) OS, secondary analysis, NMA BRAF-mutant subgroup; d PFS, secondary analysis, NMA BRAF-mutant subgroup; e OS, secondary analysis, MAIC; (f) PFS, secondary analysis, MAIC. DAB + TRAM dabrafenib plus trametinib, ENCO + BINI encorafenib plus binimetinib, IPI ipilimumab, NIVO + IPI nivolumab plus ipilimumab, MAIC matching-adjusted indirect comparison, NMA network meta-analysis, OS overall survival, PEMBRO pembrolizumab, PFS progression-free survival, VEM + COBI vemurafenib plus cobimetinib
The primary ITC analysis pooled trials with patients who had either BRAF-mutant disease only, BRAF wild-type disease only, or both BRAF-mutant and BRAF wild-type diseases, based on the assumption that there is no effect modification by BRAF status for non-BRAF-targeted therapies. Two secondary analyses based on different specifications for the ITCs were also conducted to examine the sensitivity of the indirect comparison when restricted to the BRAF-mutant population only. The first analysis was similar to the NMA fractional polynomial framework in the primary analysis, but it was restricted to BRAF-mutant subgroup data (Fig. 1c, d). PEMBRO was excluded from this analysis because of a lack of published, detailed data for the BRAF-mutant subgroup. The second analysis was a matching-adjusted indirect comparison (MAIC) that was conducted for the BRAF + MEK inhibitor combination comparisons only (Fig. 1e, f). For the MAIC analyses, the reference treatment was NIVO + IPI. ITC parameters for the primary and secondary analyses are provided in the Electronic Supplementary Appendix (Sections 2–4).
Reference survival curves for IPI monotherapy (primary NMA and NMA among the BRAF-mutant subgroup only) and NIVO + IPI (MAIC) were estimated based on data from the 60-month database lock (i.e., time of data analysis) of the CheckMate 067 trial. A standard parametric survival analysis of OS and PFS data was conducted. Evaluation of log–log plots, Schoenfeld residuals, and quantile–quantile plots indicated a violation of the proportional hazard and accelerated failure time assumptions for both endpoints; therefore, independent models were fitted for each treatment arm. In this approach, the hazard functions of the interventions in the trial were modeled using standard parametric survival distributions (exponential, Weibull, Gompertz, log-normal, log-logistic, and generalized gamma). The best fitting distributions were selected based on statistical goodness-of-fit tests (Akaike information criteria/Bayesian information criteria) and visual inspection of the curves for clinical plausibility and consistency of the OS and PFS curve combinations (Electronic Supplementary Appendix, Section 5) [29]. For the IPI reference, log-normal distribution was selected for OS, and the generalized gamma distribution was selected for PFS. For the NIVO + IPI reference curves for the MAIC analysis, the Gompertz distribution was selected for both OS and PFS.
In all analyses, extrapolated OS rates were adjusted with age- and sex-specific, annual mortality rates taken from life tables prepared by the Centers for Disease Control and Prevention [30]. A starting age of 60 years was applied, corresponding with the mean age of patients at randomization in CheckMate 067.
2.4 Costs
Costs included those associated with drug acquisition, drug administration, disease management, and toxicity. Treatment-specific drug acquisition and administration costs per month were based on the number of treatment cycles per month, dosage per administration, and cost per package. NIVO is administered as a flat dose of 240 mg every 2 weeks (for both monotherapy and for maintenance with the NIVO + IPI regimen). The dosage required for other infusion therapies (i.e., IPI and PEMBRO) was estimated using a mean patient weight of 79.2 kg and a body surface area of 1.91 m2 based on the characteristics of patients in CheckMate 067. In the base-case analysis, leftover vials were assumed to be wasted (i.e., costs were estimated based on the minimum number of vials required). The recommended oral doses of BRAK + MEK inhibitor combinations were used in the cost calculations. The calculated cost per month for each treatment is provided in Table 1.
Treatment-specific healthcare resource use associated with disease management prior to and after progression were sourced from previously published economic models and adjusted to 2020 US dollars (US$) [31, 32]. Disease management costs included healthcare encounters outside of regularly scheduled administration encounters, such as concomitant medications, hospitalizations, laboratory tests, procedures, surgeries, and consultations, and were originally costed using Medicare fee schedules [31, 32]. These recurring, monthly costs were assigned based on progression status (pre- and post-progression) and treatment status (on-treatment and off-treatment).
AE costs were estimated by combining the rate of grade 3 or higher AEs reported in published RCTs with average event costs sourced from published literature or the Healthcare Cost and Utilization Project—National (Nationwide) Inpatient Sample for 2016. AE frequencies and corresponding AE costs are provided in the Electronic Supplementary Appendix (Section 6).
After progression following first-line treatment, it was assumed that for each comparator a proportion of patients would receive subsequent active systemic therapy, whereas the remainder of the patients having progressive disease were assumed to proceed with no active treatment. In the base-case analysis, the distribution of subsequent therapies received was based on their reported shares from CheckMate 067 (NIVO + IPI, NIVO, IPI, and PEMBRO [assumed to be the same as NIVO]) and pooled estimates from COMBI-d and COMBI-v (for DAB + TRAM, assumed to be the same for VEM + COBI and ENCO + BINI) (Table 2) [6, 15]. Mean duration of subsequent therapy (either overall or by individual therapy received) has not been reported in any of the relevant clinical trials. Therefore, a mean duration of subsequent treatment of 4.9 months was assumed for all treatments (except for IPI, which was capped at 2.4 months, the mean duration from CheckMate 067) based on the estimated mean PFS reported for second-line therapy after anti-programmed death (PD)-1 treatment (NIVO or PEMBRO) reported in the literature [33]. All costs were discounted at a rate of 3% per year in alignment with US-based recommendations [28]. Results are presented in 2020 US$.
2.5 Treatment Duration
Given that NIVO is administered until progression (or beyond) in RCTs per protocol, it is not clear from the available data what the optimal therapy duration is in clinical practice, but US guidelines indicate that in most patients who achieve responses and discontinue anti–PD-1 monotherapy after 2 years, responses are maintained up to 2 years later [34]. The model assumed that treatment duration was dictated by the PFS curve (i.e., treatment until progression), with a stopping rule applied. For NIVO + IPI, NIVO, and PEMBRO, a 2-year stopping rule was applied as a base-case assumption according to the guidelines noted above. For the BRAF + MEK inhibitor combinations, the median PFS reported in the literature was applied as a maximum treatment duration (Table 1). For IPI, which has a fixed maximum treatment duration of four doses, a mean of 3.5 doses (sourced from CheckMate 067) was applied to all patients. Survival extrapolations were not altered for patients who discontinued treatment due to the stopping rule. The resulting mean durations for each treatment in the primary analyses are provided in Table 1.
NIVO + IPI was administered in an induction phase during which patients received both drugs, with a maximum of four IPI doses. This was followed by a maintenance phase of NIVO monotherapy. In CheckMate 067, 43% of NIVO + IPI patients discontinued both treatments before completing induction [6]. OS and PFS for the subgroup of patients who discontinued treatment during induction were found to be similar to those of the overall population [6]. To account for this, the model incorporated the percentage of patients who completed induction by response status at 4 months. The model then applied a fixed time on treatment for patients who did not complete induction and a treat-to-progression approach (with a 2-year stopping rule) for those who completed induction, went on to maintenance therapy with NIVO, and remained on treatment until progression.
This approach to modeling treatment duration—based on the PFS curves combined with a stopping rule—was necessary in this analysis because detailed time-to-discontinuation data or information on mean duration were not available for all competing treatments in the clinical trials. In contrast, the PFS curves afforded a consistent approach because, with the exception of IPI, these treatments are generally administered until progression. To test the impact of this assumption on the results, we also applied a 5-year stopping rule to I-O therapies as a sensitivity analysis.
2.6 Utilities
Utility estimates in the model were based on an analysis of EuroQol-5 dimensions-3 levels (EQ-5D-3L) utility scores collected in CheckMate 067 using the US time trade-off tariff [35]. Longitudinal multivariate models were explored to determine significant predictors of utility over time. Progression status, baseline EQ-5D-3L, month prior to death, and assigned treatment were all significant predictors of utility over time. Based on these analyses, the pre-progression utility weight was 0.842 and the post-progression weight was 0.819, with a decrement for the cycle prior to death of 0.082.
Grade 3 or higher AE-related disutilities were also included in the calculation of QALYs, depending on the setting (outpatient/1-day inpatient, − 0.13; inpatient > 1 day, − 0.17 [Electronic Supplementary Appendix, Section 6]). The decrements were sourced from a cross-sectional study eliciting toxicity-related disutilities (via a standard gamble approach) in patients with advanced melanoma [36].
2.7 Sensitivity Analyses
A probabilistic sensitivity analysis (PSA) was performed for 1000 replications, which was sufficient to achieve stabilization and to explore the impact of parameter uncertainty in the model. The inputs and assumptions that were tested in the PSA included survival parameters for OS and PFS reference curves and NMA parameters (variance-covariance matrix using the Cholesky decomposition), frequency of AEs (assumed as beta distribution), disease management costs (assumed as gamma distribution with 20% standard error), and health-state utility estimations (assumed as beta for the intercept and baseline coefficients, and normal for the difference to post-progression and death). Net monetary benefit acceptability curves (NBAC) summarized the PSA results for various willingness-to-pay ceiling ratios. A full listing of parameters used in the PSA is provided in Electronic Supplementary Appendix (Section 7). Deterministic sensitivity analyses were also conducted to assess the impact of selected individual inputs on ICUR estimates, including discount rates, utility inputs, and AE costs (discount rates were varied from 0 to 6%; all other inputs varied ±20% from their baseline values). Tornado plots summarizing the results from these sensitivity analyses are provided in the Electronic Supplementary Appendix (Section 8). It is important to emphasize that survival parameters and treatment-specific disease management costs were varied in the PSA only. To test the impact of assumptions made in the model, including survival extrapolation and treatment cap, scenario analyses were also conducted as follows:
-
selecting secondary (i.e., second best-fitting) parametric distribution for IPI reference curve;
-
setting 5-year maximum treatment duration for I-O therapies (NIVO + IPI, NIVO, PEMBRO).
3 Results
In the primary analysis, NIVO + IPI was estimated to have the longest survival and the highest total costs versus all comparators, accruing 6.99 LYs, 5.70 QALYs, and $469,469 over the 30-year model time horizon (Table 3). The two-largest single-most cost components for NIVO + IPI, as well as other comparators, were treatment and subsequent treatments. The incremental cost-utility of NIVO + IPI versus the BRAF + MEK inhibitor combinations ranged from $2130 per QALY (ENCO + BINI) to $26,876 per QALY (DAB + TRAM) (Table 4). Comparisons versus I-O monotherapies ranged from $6119 per QALY (IPI) to $76,169 per QALY (NIVO) (Table 4).
Results of the secondary analysis when applying the NMA to the BRAF-mutant subgroup were generally aligned with the primary all-comer analysis, with the exception of the ENCO + BINI comparison, in which the ICUR increased to $14,708. In the secondary analysis based on the MAIC versus BRAF + MEK inhibitor combinations, ICURs were lower than those for the all-comer analysis due to higher incremental survival benefit and lower incremental costs.
In scenario analyses, applying alternative reference curves for IPI, ICURs for NIVO + IPI increased slightly for all comparisons versus the primary analysis, but all of the ICURs still fell below a willingness-to-pay threshold of $100,000 per QALY (Table 5). ICURs increased more significantly when the stopping rule for I-O therapies was increased from 2 to 5 years, which resulted in higher incremental costs for NIVO + IPI, with ICURs falling between $102,832 and $129,550 per QALY gained for comparisons against NIVO, DAB + TRAM, and ENCO + BINI.
Findings from 1000 replications of the model for PSA were summarized via NBACs presented in Fig. 2. For each of the comparators, the proportion of replications in which treatment provided the greatest net monetary benefit for a given willingness-to-pay threshold is presented. NIVO + IPI provided the greatest net monetary benefit in most instances for willingness-to-pay thresholds greater than $80,000 per QALY.
Net benefit acceptability curves, primary analysis. a2020 US$. DAB + TRAM dabrafenib plus trametinib, ENCO + BINI encorafenib plus binimetinib, IPI ipilimumab, NIVO nivolumab, NIVO + IPI nivolumab plus ipilimumab, PEMBRO pembrolizumab, QALY quality-adjusted life-years, US$ US dollars VEM + COBI vemurafenib plus cobimetinib
4 Discussion
This analysis estimated the cost-utility of NIVO + IPI versus other first-line treatments for advanced melanoma based on time-varying treatment effects that were estimated from an ITC synthesizing all available long-term data. At the time of this analysis, data from CheckMate 067 had reached a minimum of 5 years of follow-up, with median OS not yet reached for NIVO + IPI [6]. This combination was found to be cost effective versus I-O monotherapy and BRAF + MEK inhibitor combinations, with all base-case ICURs falling below $100,000 per QALY (less than three times gross domestic product per capita in the US = $195,000) [28, 37]. These results highlight the additional clinical and economic value of adding IPI to NIVO as first-line treatment for advanced melanoma.
In this analysis, OS and PFS were estimated using a time-varying treatment effect based on various ITC methods. Across all ITC analyses, HRs for NIVO + IPI compared with other treatments varied significantly with time, generally showing limited or no survival benefit in the first 3–9 months of follow-up, followed by steady decreases to statistically significant HRs over time for most comparisons. With a more traditional constant HR approach, the survival curves would not have adequately captured the existing evidence on the survival experience with both BRAF inhibitors and I-O therapies. Using the time-varying HR estimates, the modeled curves have the expected plateau shape for the I-O therapies, as demonstrated in the 5-year data for IPI from pooled phase II and III studies [19] and for NIVO + IPI from CheckMate 067 [6]. Conversely, the primary analyses in the current report potentially underestimate the performance of the BRAF + MEK inhibitor combinations. In the MAIC secondary analyses, in which the NIVO + IPI data were reweighted to match the average baseline characteristics from the individual BRAF + MEK inhibitor trial populations, there was a more gradual decline in the estimated HR for NIVO + IPI versus the BRAF + MEK inhibitor combinations than noted in the overall population NMA or the BRAF-mutant subgroup NMA.
Estimations of the reference OS and PFS curves for IPI (and NIVO + IPI in the MAIC analysis) were based on CheckMate 067 data with 5 years of follow-up. The best-fitting parametric distributions were selected based on statistical metrics of goodness-of-fit, visual inspection of the extrapolated curves, and plausibility of the combined expressions of OS and PFS within a given treatment arm. Although several distributions provided similar fits for the observed data, there was considerable variability in the extrapolated tails beyond the study follow-up. This is not uncommon given the different distributional assumptions in the shape of the underlying hazard functions. When secondary distribution selections were tested in sensitivity analysis, while estimates of total QALYs and costs by treatment arm shifted, the incremental costs and QALY gains by NIVO + IPI versus the comparators were not found to be particularly sensitive to the choice of the survival curves extrapolating these data.
A key driver in these results was the cost of subsequent therapy (i.e., second-line systemic therapy). NIVO + IPI had the lowest costs associated with subsequent therapy and was the only treatment in which subsequent treatment costs were substantially lower than the costs associated with initial therapy, which helps offset its higher costs in that category. This analysis did not specifically model treatment sequencing, as has been done in other economic evaluations of patients with metastatic melanoma [31, 32] in which patients were assumed to continue only with a single choice of subsequent treatment in each sequence. OS estimates from the trials informing this analysis were likely influenced by the receipt of subsequent therapies after treatment discontinuation, and our analyses attempted to account for the costs associated with that mix of subsequent treatments, rather than modeling and providing a comparison of each individual treatment sequence. For this reason, subsequent treatment costs were based on the distributions reported in the CheckMate 067 and COMBI-d/COMBI-v trials. Trial data documenting the actual duration of individual subsequent therapies would have yielded a more precise account of the estimated subsequent therapy costs. Since such data were not available from CheckMate-067 or from the competitor trials, an assumed identical mean duration of 4.9 months for all treatments (except IPI) was utilized instead. This duration was based on mean second-line PFS data for patients previously treated with PD-1 therapies reported by Zimmer et al. [33]. The median PFS from this study is broadly aligned with similar second-line PFS estimates from the literature based on real-world data [38]. Despite the limitations in our approach to estimating costs related to subsequent treatments, the economic evaluations presented here are generally consistent with the findings from a prior economic evaluation based on a treatment sequencing analysis, which demonstrated that first-line therapy with NIVO + IPI (followed by BRAF + MEK inhibitor therapy) was most cost effective at thresholds greater than $80,000 per QALY [32].
Additional limitations of this study should be noted. There are limited real-world data on the duration of first-line treatment for NIVO + IPI in clinical practice. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) state that the optimal duration of anti–PD-1 therapy is currently unknown, but indicate that most patients who respond and discontinue anti–PD-1 monotherapy after 2 years maintain the response to treatment [34]. A recent retrospective chart review estimated that mean first-line treatment durations ranged from 45 to 57 weeks for I-O therapies and were 45 weeks for a BRAF + MEK inhibitor regimen [39]. In our primary analysis, patients were treated until progression with a treatment-specific maximum treatment duration unless they experienced prior progression. The effective mean treatment durations from the base-case stopping rules presented in Table 1 are slightly lower, but are still broadly aligned with these real-world estimates (40–53 weeks [9–12 months] for I-O therapies excluding IPI, and 34–36 weeks [8–9 months] for BRAF + MEK inhibitor combinations). This comparison was verified by the chart review noting particularly that the efficacy observed with all treatments in their study was slightly higher than reported treatment durations in clinical trials [39].
Without a treatment cap, an estimated proportion of patients could potentially receive lifelong treatment (i.e., full-time horizon of 30 years), which would not be a realistic representation of real-world practice for any treatment, particularly for NIVO + IPI. In CheckMate 067, most patients treated with NIVO + IPI discontinued treatment prior to completing the IPI induction phase, and yet still experienced a durable response. At a median follow-up of 12 months, 30% and 37% of patients in the NIVO + IPI and NIVO arms remained on treatment, respectively [5]; at 5 years, the percentage of patients who were still on treatment fell to 8% and 18% (among those still alive at 5 years), respectively [6]. Meanwhile, at the 5-year cut-off, 74% of patients in the NIVO + IPI arm and 58% of those in the NIVO arm who were still alive had not received any subsequent therapy [6]. Furthermore, the median treatment-free interval following NIVO + IPI therapy was reported to more than 18 months [6]. While this model did not alter survival projections for patients who discontinued treatment due to a stopping rule (because there were no clinical data available to support such an adjustment), data from CheckMate 067 provide supporting evidence that this is not an unreasonable assumption. In a more conservative testing scenario including patients treated with I-O therapy up to 5 years (keeping the maximum duration for BRAF + MEK inhibitors at their base case of approximately 1 year), results maintained NIVO + IPI as a highly cost-effective treatment option.
Lastly, it should be noted that given the limitations of the KEYNOTE-006 trial, the survival projections for PEMBRO were potentially underestimated in the base case because they are ultimately based on a mixed first- and second-line treatment population. As such, comparisons of NIVO + IPI versus PEMBRO should be interpreted with caution.
5 Conclusions
Despite the lack of head-to-head evidence comparing NIVO + IPI with BRAF + MEK inhibitor combinations at the time of this analysis, robust ITCs have shown NIVO + IPI to have a sustained survival benefit in both all-comer and BRAF-mutant subgroups. This analysis builds on these findings, showing that, in addition to sustained clinical benefit, NIVO + IPI is a cost-effective option in the US for these patients compared with other first-line treatments at a willingness-to-pay threshold of $100,000 per QALY.
References
American Cancer Society. Cancer Statistics Center. Melanoma of the skin. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Melanoma%20of%20the%20skin. Accessed 7 June 2021.
Howlader N, et al. (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD; based on November 2012 SEER data submission, posted to the SEER web site, April 2013. https://seer.cancer.gov/archive/csr/1975_2010/. Accessed 22 May 2021.
Khair DO, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453. https://doi.org/10.3389/fimmu.2019.00453.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous V.2.2021. ©National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Accessed 6 July 2021. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. https://doi.org/10.1056/NEJMoa1504030.
Larkin J, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46. https://doi.org/10.1056/NEJMoa1910836.
Wolchok JD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–37. https://doi.org/10.1200/JCO.21.02229.
Hodi FS, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1158–68. https://doi.org/10.1016/S1470-2045(16)30366-7.
Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. https://doi.org/10.1056/NEJMoa1503093.
Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–51. https://doi.org/10.1016/S1470-2045(19)30388-2.
Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Larkin J, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76. https://doi.org/10.1056/NEJMoa1408868.
Ascierto PA, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–60. https://doi.org/10.1016/S1470-2045(16)30122-X.
Long GV, et al. Overall survival in COMBI-d, a randomized, double-blinded, phase III study comparing the combination of dabrafenib and trametinib with dabrafenib and placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma. J Clin Oncol. 2015;33(15, Suppl). https://doi.org/10.1200/jco.2015.33.15_suppl.102.
Robert C, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–36. https://doi.org/10.1056/NEJMoa1904059.
Robert C, et al. COMBI-v: a randomised, open-label, phase III study comparing the combination of dabrafenib (D) and trametinib (T) with vemurafenib (V) as first-line therapy in patients (pts) with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma. Ann Oncol. 2014;25(Suppl 4):v1–41. https://doi.org/10.1093/annonc/mdu438.39.
Robert C, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable of metastatic BRAF V600E/K-mutant cutaneous melanoma. Ann Oncol. 2016;27(Suppl 6):VI575. https://doi.org/10.1093/annonc/mdw435.37.
Dummer R, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–15. https://doi.org/10.1016/S1470-2045(18)30142-6.
Schadendorf D, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–94. https://doi.org/10.1200/JCO.2014.56.2736.
Mohr P, et al. Long-term efficacy of combination nivolumab and ipilimumab for first-line treatment of advanced melanoma: a network meta-analysis [abstract 1347P]. Ann Oncol. 2019;30(Suppl 5): v550. https://doi.org/10.1093/annonc/mdz255.036.
Tarhini AA, et al. A matching-adjusted indirect comparison of combination nivolumab plus ipilimumab with BRAF plus MEK inhibitors for the treatment of BRAF-mutant advanced melanoma. ESMO Open. 2021;6(2):100050.
Gorry C, McCullagh L, Barry M. Transferability of economic evaluations of treatments for advanced melanoma. Pharmacoeconomics. 2020;38(2):217–31. https://doi.org/10.1007/s40273-019-00860-y.
Jensen IS, et al. Evaluating cost benefits of combination therapies for advanced melanoma. Drugs Context. 2016;5:12297. https://doi.org/10.7573/dic.212297.
Oh A, et al. Cost-effectiveness of nivolumab-ipilimumab combination therapy compared with monotherapy for first-line treatment of metastatic melanoma in the United States. J Manag Care Spec Pharm. 2017;23(6):653–64. https://doi.org/10.18553/jmcp.2017.23.6.653.
Hirst A, et al. Cost-effectiveness of nivolumab in combination with ipilimumab in first-line treatment of advanced melanoma in three European countries using 28-month overall survival from Checkmate 067 [abstract only]. Value Health. 2017;20(9):A432. https://doi.org/10.1016/j.jval.2017.08.197.
Sabater J, et al. Cost-effectiveness of nivolumab + ipilimumab in first-line treatment of advanced melanoma: analysis using 28-month overall survival from CheckMate 067 [abstract 2440]. Ann Oncol. 2017;28(Suppl 5):v396. https://doi.org/10.1093/annonc/mdx375.003.
Paly VF, et al. Economic evaluation of nivolumab combined with ipilimumab in the first-line treatment of advanced melanoma in Japan. J Med Econ. 2020;23(12):1542–52. https://doi.org/10.1080/13696998.2020.1830781.
Institute for Clinical and Economic Review. 2020-2023 Value Assessment Framework. 31 January 2020. https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 22 May 2021.
Latimer NR. Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013;33(6):743–54. https://doi.org/10.1177/0272989X12472398.
Arias E, Xu J, for the Division of Vital Statistics. United States life tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–65.
Tarhini A, et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: a cost–effectiveness analysis. Immunotherapy. 2018;10(14):1241–52. https://doi.org/10.2217/imt-2018-0085.
Tarhini A, et al. Clinical and economic outcomes associated with treatment sequences in patients with BRAF-mutant advanced melanoma. Immunotherapy. 2019;11(4):283–95. https://doi.org/10.2217/imt-2018-0168.
Zimmer L, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55. https://doi.org/10.1016/j.ejca.2017.01.009.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous. NCCN Evidence Blocks V.2.2021. ©National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Accessed July 6, 2021. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20. https://doi.org/10.1097/00005650-200503000-00003.
Beusterien KM, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101(3):387–9. https://doi.org/10.1038/sj.bjc.6605187.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35. https://doi.org/10.1016/j.jval.2016.02.017.
Cybulska-Stopa B, et al. Real-world treatment practice in patients with advanced melanoma. Contemp Oncol (Pozn). 2020;24(2):118–24. https://doi.org/10.5114/wo.2020.97607.
Tarhini A, et al. Treatment patterns and outcomes for patients with unresectable stage III and metastatic melanoma in the USA. J Comp Eff Res. 2019;8(7):461–73. https://doi.org/10.2217/cer-2019-0003.
Acknowledgements
The authors thank Brian Stwalley and Cho-Han Lee for their contributions to data interpretation. Editorial assistance was provided by Mark Palangio and Michele Salernitano of Ashfield MedComms, an Inizio company, funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Bristol Myers Squibb.
Conflicts of interest
Timothy Baker and Victoria Federico Paly are employees of ICON plc, which received funding from Bristol Myers Squibb for this work. Helen Johnson received financial payment from Bristol Myers Squibb for this work. Srividya Kotapati and Murat Kurt are employees of Bristol Myers Squibb. Andriy Moshyk and Melissa Hamilton are employees and shareholders of Bristol Myers Squibb.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Code availability
Not available.
Authors’ contributions
Conception or design: TB, HJ, SK, AM, VP. Data acquisition: TB, HJ, SK, AM, VP. Data analysis: TB, HJ, SK, AM, MH, MK, VP. Data interpretation: All authors.
Additional information
Helen Johnson was an employee at Bristol Myers Squibb at the time of analysis.
Victoria Federico Paly was an employee at ICON plc at the time of analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Baker, T., Johnson, H., Kotapati, S. et al. Cost-Utility of Nivolumab Plus Ipilimumab in First-Line Treatment of Advanced Melanoma in the United States: An Analysis Using Long-Term Overall Survival Data from Checkmate 067. PharmacoEconomics Open 6, 697–710 (2022). https://doi.org/10.1007/s41669-022-00348-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00348-0