Abstract
Objective
This study aimed to analyze the healthcare resource use (HCRU) and associated costs of multiple myeloma (MM) using German claims data.
Methods
Anonymized claims data from one of the largest sickness funds in Germany were analyzed. Costs and HCRU were calculated from the perspective of the statutory health insurance. To analyze MM-associated incremental costs in a most recent calendar period for an overall MM population (31 March 2018–31 March 2019), a prevalent cohort of MM patients (continuously insured during 01 January 2010 until 31 March 2019 or death; two or more outpatient and/or one inpatient MM diagnoses [ICD-10: C90.0-] and alive on 31 March 2018) was compared with a control group (not diagnosed with MM) employing propensity-score matching. Additionally, to describe MM-associated HCRU and costs for treated patients per line of treatment (lot), a cohort of newly treated patients was considered (≥ 12 months’ pre-index period without MM treatment). Therapy lines were determined based on observed days of medication supply, treatment switches, and treatment discontinuations.
Results
Overall, 2523 prevalent MM patients (52.0% female, mean age: 71.3 years) and 1673 newly treated MM patients (50.2% female, mean age: 73.0 years) met the selection criteria and were included in the analyses. After matching, a non-MM counterpart could be identified for 2474 prevalent out of 2523 MM patients. MM-associated incremental HCRU was characterized by an increased number of hospitalizations and a higher number of outpatient specialist visits (per patient-year [ppy] 0.48 additional hospitalizations and 3.80 additional specialist visits; p < 0.001), being also drivers of the associated total incremental add-on costs (15,381.09 € ppy, p < 0.001). Among newly treated patients, total direct costs ppy increased as patients received subsequent treatments (1st lot: 67,681,55 €; 4th lot+: 114,934.01 €), driven by outpatient MM prescriptions (1st lot: 28,692.32 €; 4th lot+: 62,980.72 €).
Conclusion
The economic burden of MM is driven by outpatient prescriptions, inpatient hospitalizations, and outpatient specialist visits. Treatment and overall costs increase substantially when patients move to later lines of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multiple myeloma (MM) has a significant economic burden in Germany. This study showed total all-cause direct costs of 22,847.64 € in a cross-sectional sample of patients with MM, which was three times higher than the costs observed for a matched control group without MM. |
In a longitudinal observation of identified newly-treated MM patients, it could be seen that the all-cause and primarily MM-related total direct costs per patient-year increased as patients received subsequent treatments. |
Hospital stays and outpatient drug prescriptions were identified as the main cost drivers. |
1 Introduction
Multiple myeloma (MM) is a type of bone marrow cancer caused by malignant plasma cells. Globally, the annual incidence of MM is estimated to be 6–7 cases per 100,000 persons [1], accounting for 1% of all cancer [2]. Similarly, in Germany, it is among the 20 most common malignancies with an estimated 7000 new cases every year and a 5-year prevalence of nearly 22,400 cases [1, 3].
The natural course of MM is characterized by a high risk of progression, with patients becoming less responsive to treatment with each relapse. Following a subsequent relapse, patients have reduced health-related quality of life (HRQoL) due to not only disease progression but also comorbidities [4].
Although MM is currently incurable, the treatment landscape is rapidly evolving, and improvements in overall survival (OS) have been observed over recent years [5, 6]. These are associated with the introduction and widespread use of multiple novel agents and regimens [7], as well as with the emerging treatment paradigm of continuous therapy or long-term maintenance therapy [8,9,10], an approach that has been shown in clinical trials to offer prolonged survival versus fixed-duration or shorter-term therapeutic approaches [9,10,11,12]. In 2010, the main novel agents approved for use were bortezomib, lenalidomide, and thalidomide [13,14,15]. Since then, ten novel agents (i.e., pomalidomide, carfilzomib, panobinostat, daratumumab, ixazomib, elotuzumab, selinexor, belantamab mafodotin, isatuximab, ide-cel) have been approved [16].
Given this intricate disease course and the relatively wide array of therapeutic alternatives, it is increasingly important to understand the real-world economic burden of MM. Although several studies have demonstrated the costs associated with MM in the US and several European countries, far less is known about the economic burden associated with the real-world treatment of MM in Germany [17,18,19,20]. This study aims to bridge this gap by analyzing the healthcare resource utilization and costs in patients with MM, using claims data from a statutory sickness fund in Germany.
2 Methods
2.1 Data Source
This study used healthcare claims data from a statutory sick fund (AOK PLUS) in Germany for the period 01 January 2010 to 31 March 2019. AOK PLUS covers nearly 3.4 million insured persons in the regions Saxony and Thuringia, which corresponds to approximately 50% of the regional population and represents 4.4% of the nationwide statutory insured population. The data include information on all the claims from inpatient and outpatient care as well as the number of days absent from work, sociodemographic characteristics of the enrolled persons, and all-cause mortality.
2.2 Study Population
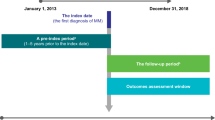
Within the study, two cohorts were investigated: (i) a cohort of prevalent patients and (ii) a cohort of newly treated patients. The scheme of patient selection for both cohorts is depicted in Fig. 1.
We analyzed a prevalent cohort of MM patients in a most recent calendar period for two reasons: (i) from a payor perspective, it might not only be important to understand what the healthcare resource use (HCRU) and associated costs of patients currently receiving treatment are but also to capture respective numbers for patients in remission or, ideally, for an ‘average MM cohort’. These numbers should be generated in the most recent year observable in the dataset as the treatment paradigm changed substantially over the last years. Therefore, the cohort of prevalent patients comprised continuously insured patients who were alive on 31 March 2018 with at least two confirmed outpatient MM diagnoses (ICD-10 code C90.0-) in two different quarters that were no more than 12 months apart or at least one inpatient MM diagnosis between 01 January 2010 and 31 March 2018. The index date for this cohort was defined as 31 March 2018, after which the outcomes were observed until 31 March 2019 (or until death, whatever came first).
The cohort of newly treated patients was made up of continuously insured patients with at least two confirmed outpatient MM diagnoses in two different quarters within 12 months or at least one inpatient MM diagnosis before the start of their MM treatment, who initiated an MM-related treatment after 31 December 2010. The date of the first observed MM treatment was considered as the index date after a minimum of 12 months of a treatment-free period.
2.3 Outcomes and Analyses
Three sets of analyses were performed in this study: (i) description of baseline characteristics across the two cohorts, (ii) analysis of MM-associated incremental economic burden in the prevalent MM cohort, and (iii) description of all-cause and primarily MM-related HCRU and costs in successive therapy lines in MM treatment starters.
2.3.1 Patient Characteristics
Patient characteristics were descriptively analyzed for both cohorts, referring to the respective index date or the 12-month pre-index period. Age, gender (both at index date), and comorbidities described by the Charlson Comorbidity Index (CCI [21]; based on diagnoses observed in the 12-month pre-index period; Supplementary Table 1, see electronic supplementary material [ESM]) were reported. Frequency analysis was applied for categorical variables, reporting the number and percentage of patients for each category. For continuous variables, summary statistics, including mean and standard deviation (SD), were reported.
2.3.2 Assessment of Healthcare Resource Use (HCRU) and Cost
The utilization of healthcare resources was evaluated in terms of the number of outpatient visits divided into General Practitioner (GP) or specialist visits, number of inpatient visits, number of prescriptions, number of rehabilitation, and number of days absent from work. Outpatient visits were approximated by counted dates of invoiced services. An item was defined as primarily MM-related HCRU if the respective claim was directly linked to the MM diagnosis (i.e., hospitalizations with MM as the main diagnosis, outpatient visits with a documented MM diagnosis, days absent from work due to MM, and outpatient prescriptions of MM-related drugs as listed in Supplementary Table 2).
The costs related to the HCRU were calculated on an annual scale for each individual and reflected the payer perspective except for the indirect costs of days absent from work, where a societal perspective was considered. Unit costs for all HCRU categories referred to values at the corresponding date of healthcare resource. The claims from inpatient care, covering all performed services and administered pharmacological treatments during hospitalization stays, are valued based on the diagnosis-related groups’ (DRG) reimbursement codes. Reimbursement of services in the outpatient care setting in Germany is regulated by the Uniform Valuation Scheme (EBM) [22]. Within the EBM scheme, the outpatient services are valued based on pre-defined weighted points that were multiplied by a uniform orientation value. In this analysis, the values used ranged from 0.03055 € in 2010 and 2011 to 0.1082 € in 2019. The outpatient prescriptions, on the other hand, were valued based on the pharmacy retail price (‘Apothekenabgabepreis’). Indirect costs due to sick leave days were calculated by multiplying the number of sick leave days by the mean daily loss of productivity for a working person in Germany in the related calendar year [23].
2.3.3 Multiple Myeloma (MM)-Associated Incremental Economic Burden
Based on the most recent period (31 March 2018–31 March 2019) and addressing an ‘average’ prevalent MM population, propensity score matching was applied to identify the ‘non-MM healthy’ counterparts of the prevalent cohort of MM patients. The underlying assumption was that the difference between the economic burden of MM patients over and above the economic burden of their non-MM counterparts would yield the overarching economic burden associated with MM.
The ‘healthy’ control group (non-MM patients) was identified in the sickness fund dataset by identifying all patients without cancer, sepsis/shocks, infections caused by opportunistic pathogens, and without any HIV/AIDS diagnosis. To estimate the propensity scores, logistic regression was run with belonging to the MM versus non-MM group as the dependent variable and the above-outlined patient characteristics as covariates (Supplementary Table 2, see ESM). After the propensity scores were estimated, the prevalent cohort of MM patients was one-to-one matched to non-MM patients, following a caliper rule of < 0.001.
After the matching was conducted, the per patient-year (ppy) HCRU items and related costs of the two groups observed from 31 March 2018 until 31 March 2019 or the date of death were compared.
MM-associated HCRU and costs were calculated as the differences in the respective ppy HCRU and cost outcomes (i.e., incremental HCRU and costs between the matched MM and the control group). The group differences in the costs incurred were evaluated based on an incremental cost approach using the Bang and Tsiatis estimator with possibly censored data [24]. Costs and HCRU event counts ppy, their increments, rate ratios, as well as the associated p-values were reported for each outcome variable.
2.3.4 All-Cause and (Primarily) MM-Related HCRU and Costs in Successive Therapy Lines
For newly treated MM patients, we assessed all-cause and primarily MM-related economic burden over successive treatment lines. For this, the cohort of newly treated MM patients was assigned to successive treatment lines following a treatment algorithm stated in Table 1. The duration of the therapy lines was assessed by the dates of events starting and ending the therapy lines, as in Table 1. For each line of therapy (lot), observations were censored at the time of death, switch to the next therapy line, discontinuation of the therapy line, or at the end of the study period.
Using the guidelines on MM treatment in Germany [25, 26], the relevant summary of product characteristics, and clinical expert opinion, the following treatments were considered as MM-related treatments: chemotherapy (bendamustine, cyclophosphamide, doxorubicin, melphalan); corticosteroids (dexamethasone only if the strength stated on the pack is 20 or 40 mg per pill; proteasome inhibitors (bortezomib, carfilzomib, ixazomib); immunomodulators (lenalidomide, pomalidomide, thalidomide); monoclonal antibodies (daratumumab, elotuzumab); and histone deacetylase (panobinostat). For the Anatomical Therapeutic Chemical (ATC, outpatient) and operational and procedure (OPS, inpatient) codes, please see Supplementary Table 3 in the ESM. HCRU and cost outcomes were described for the entire cohort of newly treated patients for the first three therapy lines as well as the fourth and further lines.
2.3.5 General Considerations
In all analyses, p-values <0.05 were considered statistically significant. Statistical analyses were performed using Microsoft SQL Server 2014 (Microsoft Corporation, Redmond, WA, USA), STATA/MP 14 (StataCorp LLC, College Station, TX, USA), and Microsoft Excel.
As the study addressed a retrospective anonymized dataset, no ethical review was needed. However, the study protocol was reviewed by a scientific steering committee and the data owner (statutory health insurance, AOK PLUS). The work on the dataset conformed to all social security data protection requirements.
3 Results
3.1 Patient Characteristics
As shown in panels a and b of Fig. 2, 5711 patients were continuously insured for the entire study period and had a diagnosis of MM based on the considered diagnostic criteria. Among those, 2523 patients for the prevalent cohort and 1673 patients for the cohort of newly treated patients were ultimately included.
The baseline characteristics of these patients are summarized in Table 2. Within the prevalent cohort of MM patients, the mean age was 71.3 years, and 52.0% of the patients were female. The average level of baseline comorbidity, captured by the CCI score, was 5.9. For the cohort of newly treated MM patients, similar baseline figures were observed, where (i) the mean age was 73.0 years, (ii) 50.2% of the patients were female, and (iii) the mean CCI score was 5.2.
3.2 Incremental MM-Associated HCRU/Costs
Nearly 1,567,217 patients in the entire database were initially eligible for the non-MM ‘healthy’ control group for the purposes of propensity score matching. After the propensity scores were estimated and the previously outlined matching procedure was applied, for 2474 patients (98.1%) of the prevalent MM cohort, a ‘non-MM counterpart’ could be identified. The quality of matching was assessed by comparing standardized differences between the groups in the main characteristics (baseline age, gender, and CCI scores) before and after matching (see Supplementary Table 4 in the ESM). The characteristics were significantly different between MM and non-MM individuals before the PS matching, whereas no significant differences were observed after matching.
The results of the outcome assessment based on the matched MM and non-MM patients are shown in Table 3. The matched MM patients were observed to have significantly higher ppy inpatient hospitalizations (incremental utilizations: ∆ = 0.48), outpatient specialist visits (∆ = 3.8) and outpatient prescriptions (∆ = 7.2) compared with their non-MM counterparts in the matched control group, with rate ratios of 1.44 (p < 0.001), 1.56 (p < 0.001), and 1.22 (p < 0.001), respectively. Conversely, the incremental ppy GP visits (∆ = − 0.13) and the days of sick leaves (∆ = − 1.89) were significantly higher in the non-MM group, with the respective rate ratios of 0.97 (p = 0.016) and (p < 0.001). No significantly different incremental utilization of inpatient rehabilitations was observed across the two groups.
Associated direct costs were substantially higher for the MM group (Table 3), with add-on ppy incremental direct costs of 15,381.09 € (cost ratio = 3.06; p < 0.001). Outpatient prescriptions and hospitalizations were the main cost driver, with MM-associated incremental costs amounting to 12,261.22 € and 2,766.98 €, respectively, followed by outpatient specialist visits (ppy MM-associated incremental costs: ∆ = 359.57 €). The share of the costs of GP visits and rehabilitations in the total MM-associated incremental costs was negligible. Finally, the indirect costs of days absent from work were lower in the MM group compared with their non-MM controls by 263.53 € ppy.
3.3 All-Cause and (Primarily) MM-Related HCRU/Costs in Successive Therapy Lines
Based on the algorithm shown in Table 1, 1673 patients were identified to initiate an MM-related treatment. Out of these, 961 patients moved to 2nd lot. Subsequently, 483 of the patients on 2nd lot progressed to 3rd lot in their treatment journey. Ultimately, 254 patients were identified to have finally moved to 4th lot+. The average duration of the treatment lines was nearly 217.6 days for 1st lot, 260.2 days for 2nd lot, 226.5 days for 3rd lot, and 148.8 days for 4th lot+ (see Supplementary Table 5 in the ESM for further details as well as for the baseline characteristics and the associated regimens for each lot).
The ppy costs associated with the above-mentioned HCRU items are presented in Table 4. Overall, the total direct costs increased substantially as patients moved in their treatment journey. All-cause ppy total direct costs defined as the sum of costs of all-cause hospitalizations, outpatient visits, inpatient rehabilitations, and outpatient prescriptions were 67,681.55 € in 1st lot but increased to 114,934.01 € as patients progressed to 4th or later line. Similarly, primarily MM-related total direct costs increased from 54,355.95 € in 1st lot to 89,113.79 € in the 4th or later lines. The main cost drivers were the outpatient prescriptions, which also showed an increasing trend over the treatment journey, followed by hospitalizations. The ppy costs associated with all-cause outpatient visits (GPs and any specialists) did not differ considerably between the lines, with approximately 40% of the costs of the outpatient visits being primarily associated with MM. As rehabilitations did not play a big role in disease management across all treatment lines, the associated ppy costs were also negligible.
Finally, the number of days absent from work due to any cause or MM declined from 1st lot (all-cause: 29.1 days, MM-related: 28.2 days ppy) through the 4th lot and later lines (all-cause: 3.53, MM-related: 2.83 days ppy). Consequently, the associated indirect costs decreased over the course of treatment, as shown in Table 4.
4 Discussion
This study is the first of its kind, presenting results on both overarching incremental economic burden associated with MM patients as well as the economic burden primarily related to MM in successive therapy lines in Germany. The analyses were based on a large pool of patients (nearly 3.4 million insured persons, 4.4% of the overall German population) with relatively long data availability (01 January 2010–31 March 2019) and no data gaps due to continuous longitudinal insurance of all patients and reimbursement of all prescribed healthcare services such as outpatient visits, inpatient hospitalizations, and prescribed drugs in Germany that is close to 100% (no substantial out-of-pocket payments). This (i) ensured capturing patients from different treatment backgrounds in this fast-changing treatment landscape and (ii) facilitated observing the cohorts of interest with reasonable sample sizes despite strict eligibility criteria.
In total, 2523 prevalent MM patients could be identified. The patients were on average aged 71 years, and we observed a nearly equal distribution of female and male patients, which is in line with reports based on German-wide data [27]. We found a high MM-associated incremental economic burden, demonstrated by the use of healthcare resources and the related direct costs. Specifically, the incremental total direct costs ppy amounted to 15,381.08 €. The observed incremental economic burden was mainly driven by outpatient prescriptions, inpatient hospitalizations, and outpatient specialist visits, whereas the share of GP visits and rehabilitations in the incremental economic burden of MM was negligible. Although no previous literature on the incremental economic burden of MM could be identified, our results align with the comparative literature on the real-world treatment of MM patients in EU versus the US such that the inpatient care as well as outpatient care by specialists play a bigger role in the management of MM within the EU [27] and, hence, are expected to be associated with higher costs. It should also be noted that our incremental cost estimates were based on all prevalent MM patients irrespective of whether they received treatment or not in the observational period.
In addition to analyzing the MM-associated incremental costs, this study assessed the HCRU and costs associated with the treatment of MM patients by analyzing a cohort of patients newly starting their MM treatment throughout their therapy journey. The fast-changing treatment landscape of MM calls for a better understanding of disease progression and management in a resource-constrained environment [29, 30]. Despite this, no previous studies in Germany with a focus on understanding the economic burden over treatment patterns could be identified. However, recent US findings [18] largely align with our key finding for Germany that pharmacy costs (i.e., outpatient prescriptions) are the main cost driver and increase as patients progress to further treatment lines. In particular, the ppy costs of MM prescriptions in our study ranged from 28,692.32 € in the 1st lot to 54,432.08 € in the 3rd lot and 62,980.72 € in the 4th and later lines. Recent US findings [18] suggest US$4886 in the 1st lot and US$13,583 per patient month. However, in several US findings, the costs of MM prescriptions accounted for a smaller portion of the all-cause costs, ranging from 22.0 to 23.7% in the 1st lot and from 29.0 to 37.2% in the 3rd lot [31]. In our study, these figures were 42.4% in the 1st lot and 56.3% in the 3rd lot. This is potentially due to differences in healthcare systems, healthcare unit price differences, the treatment algorithms applied, and heterogeneity in the treatment rules across Germany and the US.
Although one study focusing on a Portuguese population [20] also demonstrated that outpatient prescriptions were the largest cost drivers in all treatment lines followed by the costs due to hospitalizations (including both the inpatient stays as well as the cost of inpatient procedures such as stem cell transplantation), the magnitude of costs are not comparable due to restrictions in the follow-up periods (24 months considered) and because reporting is on a per-patient basis.
Regarding the costs of all-cause and MM-related hospitalizations over the treatment lines, our study showed a pattern where the costs declined from the 1st lot through the 3rd lot but increased in the 4th and later lines. Although these findings might corroborate with the previous literature showing that delaying progression to successive therapy lines could result in subsequent lower costs [31], further investigation on this would be needed.
We found a low indirect economic burden to society associated with days absent from work. Two main factors could drive these results. First, as patients move to further treatment lines, they might drop out of the labor force due to disease progression. Second, the average ages in the cohorts considered in this study were 71.3 and 73.0 years. It is, therefore, expected that our target population comprises, on average, retired individuals, where sick leave and the associated forgone earnings are rather irrelevant.
We observed a substantial difference in HCRU and cost between our two cohorts of prevalent MM patients and newly treated patients. The main explanation for this is that a high percentage of the prevalent MM patients did not receive any MM treatments and did not observe any MM-related hospitalizations. We believe that this is related to the fact that the majority of observed prevalent MM patients were in remission during the observational period.
This study has several limitations common to retrospective claims data studies. As it is descriptive by nature, the potential confounding factors were not controlled. However, while analyzing the incremental MM-associated HCRU and costs, propensity score matching was implemented, where baseline sociodemographic characteristics and comorbidities were included as covariates in estimating the propensity scores to partially avail against these limitations. Furthermore, compared with other observational study designs, our study included all eligible patients (no selection bias) and covered treatment in all outpatient and inpatient sectors.
Related to conducted propensity score matching in order to derive the incremental cost of MM, it needs to be mentioned that the comorbidity index (CCI) used within the matching is impacted by the MM diagnosis itself, and thus, the matched cohort consists of patients with a similar comorbidity level that is not driven by MM but other diseases leading to high CCI. Excluding the MM component from the CCI calculation would have led to a comparison with a considerably less morbid control group and, therefore, to a higher estimate of costs attributable to MM.
Treatment lines are not explicitly captured in claims data. Therefore, an algorithm based on receipt and timing of therapy to classify treatment episodes was implemented. The assumptions made in treatment algorithms to define the line of therapy could impact subsequent HCRU and cost results as well as the duration of treatment lines. To reduce that limitation, however, the identification of a line of treatment was informed by available clinical guidelines [25], the summary of product characteristics, as well as licensing of the agents at the respective times as much as possible. Also, the definition of treatment lines was discussed with clinical experts and implemented upon their approval. In addition, the estimated costs by the line of therapy were not adjusted for inflation, so they could potentially be biased by price fluctuations between 2010 and 2018.
Finally, this study used a regional claims dataset from AOK PLUS, and, therefore, the applicability of the results to the whole of Germany could be limited. However, several previous retrospective studies [32, 33] using AOK PLUS claims data confirmed that although patient characteristics may not exactly reflect the country average, the uniform treatment rules observed throughout the country make the AOK PLUS database useful for understanding the economic burden of diseases with a high level of generalizability.
5 Conclusion
The economic burden of MM, from the perspective of both overarching and MM-specific HCRU and costs, is substantial and mainly driven by outpatient prescriptions, hospitalizations, and outpatient specialist visits as patients moved to successive treatment lines. Additional research investigating the indirect costs and burden of disease on the patient should be conducted to better understand the impact of MM. Future research should also focus on the real-world cost effectiveness of the novel treatments in this dynamic therapy landscape.
References
Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Deutsches Arzteblatt Int. 2016;113(27–28):470–6. https://doi.org/10.3238/arztebl.2016.0470.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Robert Koch Institut. Cancer in Germany 2015/2016. 12th edition. Zentrum Für Krebsregisterdaten, vol. 12, no. 1, p. 158, 2015, [Online]. https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_chapters_2015_2016/cancer_germany_2015_2016.pdf?__blob=publicationFile. Accessed 4 Feb 2020.
Gay F, Palumbo A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med Oncol. 2010;27(SUPPL):1. https://doi.org/10.1007/s12032-010-9542-z.
Regional differences in the treatment approaches for relapsed multiple myeloma: an IMF study. J Clin Oncol. https://doi.org/10.1200/jco.2012.30.15_suppl.8095. Accessed 4 Feb 2020.
Kumar SK, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–8. https://doi.org/10.1038/leu.2013.313.
Kumar SK, et al. Multiple myeloma, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(2):230–69. https://doi.org/10.6004/jnccn.2017.0023.
Guglielmelli T, Palumbo A. Multiple myeloma: is a shift toward continuous therapy needed to move forward? Expert Rev Hematol. 2015;8(3): 253–256. Expert Reviews Ltd. https://doi.org/10.1586/17474086.2015.1001360.
Palumbo A, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33(30):3459–66. https://doi.org/10.1200/JCO.2014.60.2466.
Bahlis NJ, et al. Benefit of continuous treatment for responders with newly diagnosed multiple myeloma in the randomized FIRST trial. Leukemia. 2017;31(11):2435–42. https://doi.org/10.1038/leu.2017.111.
McCarthy PL, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–89. https://doi.org/10.1200/JCO.2017.72.6679.
Benboubker L, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–17. https://doi.org/10.1056/NEJMoa1402551.
D. Meade, M. Ng, S. Alford. Lenalidomide use in multiple myeloma. 2020;38(15_suppl):e14045–e14045. https://doi.org/10.1200/JCO.2020.38.15_SUPPL.E14045.
Cavo M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia. 2006;20(8):1341–52. https://doi.org/10.1038/sj.leu.2404278.
Rajkumar SV. Thalidomide in multiple myeloma. Oncology (Williston Park). 2000;14(12 Suppl 13):11–6. https://doi.org/10.2174/138920106779116919.
Mohty M, et al. Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, noninterventional study. Clin Lymphoma Myeloma Leuk. 2018;18(10):e401–19. https://doi.org/10.1016/j.clml.2018.06.018.
Dao Tran P, Kamalakar R, Shivaji Manthena MS, Karve S. No economic burden of multiple myeloma: results from a large employer-sponsored real-world administrative claims database, 2012 to 2018 title. Blood. 2019; 134.
MacEwan JP, et al. Economic burden of multiple myeloma among patients in successive lines of therapy in the United States. Leuk Lymphoma. 2018;59(4):941–9. https://doi.org/10.1080/10428194.2017.1361035.
Neves M, et al. Multiple myeloma in Portugal: burden of disease and cost of illness. Pharmacoeconomics, no. Mm, 2021. https://doi.org/10.1007/s40273-020-00993-5.
Luís Antunes Francisco Rocha‐Gonçalves Sérgio Chacim Cinira Lefèvre Marta Pereira Sónia Pereira Aleksandra Zagorska Maria José Bento. Real‐world treatment patterns, resource use and cost burden of multiple myeloma in Portugal. Eur J Cancer Care. 2019;28(4).
Chae JW, Song CS, Kim H, Lee KB, Seo BS, il Kim D. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin Pract. 2011. https://doi.org/10.1159/000321525.
Prenzler A, Zeidler J, Braun S, von der Schulenburg J-M. Bewertung von Ressourcen im Gesundheitswesen aus der Perspektive der deutschen Sozialversicherung. PharmacoEconomics German Research Articles 2010. 2013;8(1):47–66. https://doi.org/10.1007/BF03320765.
Volkswirtschaftliche Kosten durch Arbeitsunfähigkeit 2019. 2019. https://www.baua.de/DE/Themen/Arbeitswelt-und-Arbeitsschutz-im-Wandel/Arbeitsweltberichterstattung/Kosten-der-AU/pdf/Kosten-2019.pdf?__blob=publicationFile&v=3. Accessed 15 Apr 2021.
Bang H, Tsiatis A. Estimating medical costs with censored data. Biometrika. 2000;87(2):329–43.
Kolyvanos Naumann U, Käser L, Vetter W. Multiples myelom. Praxis. 2018;96(29–30):1111–9. https://doi.org/10.1024/1661-8157.96.29.1111.
Wörmann B, et al. Leitlinie Multiples Myelom, Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V. 2018.
R. Koch-Institut. Krebs in Deutschland für 2017/2018. 2017. [Online]. http://www.krebsdaten.de/multiples-myelom. Accessed 21 Feb 2022.
Vij R, et al. Healthcare resource utilization (HCRU) in relapsed/refractory multiple myeloma (RRMM): comparison of EU and US cohorts in the preamble study. Value Health. 2016;19(7):A743. https://doi.org/10.1016/j.jval.2016.09.2265.
Dimopoulos E, Meletios A. Kastritis. Managing myeloma with limited resources in the era of novel drugs. Leuk Lymphoma. 2018;59(10):2269–2270.
Kenney J. Advances in the management of multiple myeloma: implications for payers. Health Drug Benef. 2011;4:59–60.
Arikian SR, et al. Patterns of total cost and economic consequences of progression for patients with newly diagnosed multiple myeloma. Curr Med Res Opin. 2015;31(6):1105–15. https://doi.org/10.1185/03007995.2015.1031732.
Hardtstock F, et al. Healthcare resource utilization and associated cost of patients with bone metastases from solid tumors who are naïve to bone-targeting agents: a comparative analysis of patients with and without skeletal-related events. Eur J Health Econ. 2021;22(2):243–54. https://doi.org/10.1007/s10198-020-01247-z.
Wilke T, et al. Real-world insulin therapy in German type 2 diabetes mellitus patients: patient characteristics, treatment patterns, and insulin dosage. Diabetes Metab Syndr Obes Targets Ther. 2019;12:1225. https://doi.org/10.2147/DMSO.S214288.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by GlaxoSmithKline GmbH & Co. KG.
Conflict of interest
Franz Fischer and Robert Welte are employees of GlaxoSmithKline GmbH & Co. KG. Zeki Kocaata participated in this work as an employee of Ingress-Health and has no conflict of interest to declare. Thomas Wilke is the founder of IPAM e.V. and received honoraria from Boehringer Ingelheim Pharma, Bayer Health Care, GSK, Novartis, Sanofi-Aventis, Bristol Myers Squibb, Pfizer, and other pharmaceutical companies. Hermann Einsele, who is working as a physician in the field of oncology, supported the study as a scientific advisor.
Ethics approval
As the study addressed a retrospective anonymized dataset, no ethical review was needed. However, the study protocol was reviewed by a scientific steering committee and the data owner (statutory health insurance, AOK PLUS). The work on the dataset conformed to all social security data protection requirements.
Consent to participate
As the study addressed a retrospective anonymized dataset, no informed consent of patients was needed.
Consent for publication
The study was reviewed by a scientific steering committee and the data owner and approved for publication. All co-authors consented to publish the manuscript.
Data availability
The dataset used to conduct the analysis is not publicly available. All data needed to evaluate the conclusions in the paper are present in the main text and/or the Supplementary Materials.
Code availability
All data needed to evaluate the conclusions in the paper are present in the main text and/or the Supplementary Materials. Statistical analyses were done using standard applications of the software packages mentioned in the method sections. Custom algorithms applied (i.e., identification of therapy lines) have been described in the main text and/or the Supplementary Materials.
Author contributions
Conceptualization: Z.K., T.W., F.F., R.W.; Methodology: Z.K., T.W., F.F., E.H.; Formal analysis: Z.K.; Writing–original draft preparation: T.W., Z.K.; Writing–review and editing: Z.K., T.W., F.F., R.W.,E.H; Supervision: F.F., R.W.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kocaata, Z., Wilke, T., Fischer, F. et al. Healthcare Resource Utilization and Cost of Patients with Multiple Myeloma in Germany: A Retrospective Claims Data Analysis. PharmacoEconomics Open 6, 619–628 (2022). https://doi.org/10.1007/s41669-022-00344-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00344-4