Abstract
Cluster headaches are excruciating attacks of pain that can last between 15 min and 3 h. Cluster headaches can be episodic, where patients have long pain-free intervals between attacks, or chronic, where they do not. As part of the Medical Technologies Evaluation Programme, the UK National Institute for Health and Care Excellence (NICE) considered the clinical effectiveness and cost impact of gammaCore (electroCore), a handheld, patient-controlled device used to treat and prevent cluster headache. gammaCore is a non-invasive vagus nerve stimulator, the aim of which is to modify pain signals by stimulating the vagus nerve through the skin of the neck. Evidence suggests that gammaCore reduces the intensity and frequency of cluster headaches and that the addition of gammaCore to standard care is cost saving. Therefore, the guidance published by NICE in December 2019 recommends routine adoption of gammaCore into the UK national health service. However, the guidance noted that gammaCore does not work for everyone and recommended that treatment with gammaCore should stop after 3 months in patients whose symptoms do not improve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
gammaCore reduces the frequency and severity of cluster headaches but does not work for everyone. |
There is currently no evidence of a sustained long-term benefit. Treatment with gammaCore should therefore be stopped if patients do not experience any reduction in symptoms in the first 3 months. |
gammaCore was used alongside standard treatment, so decision makers should be aware that the possible benefits achieved with gammaCore are as a result of the addition of gammaCore to current standard treatment. |
1 Introduction
This paper is part of a series that provides insight into the development of UK National Institute for Health and Care Excellence (NICE) medical technologies guidance (MTG) for new or innovative medical devices or diagnostics [1]. The aim of the guidance is to support the adoption of clinically effective and cost-saving technologies in the UK National Health Service (NHS).
Cedar is a healthcare technology research centre formed through collaboration between Cardiff and Vale University Health Board and Cardiff University. This paper summarises Cedar’s assessment report [2] and how it was used to inform the NICE MTG on gammaCore for the treatment of cluster headache (CH) [3]. The aim of this paper is to provide an insight into the development of recommendations for the use of the gammaCore device.
1.1 Background to Technology and Application
Cluster headaches are excruciating attacks of pain in one side of the head, often felt around the eye. An attack may last between 15 min and 3 h and can typically occur between one and eight times a day. Cluster headaches may be classed as episodic or chronic; people with episodic CH (eCH) have extended pain-free intervals, whereas those classed as having chronic CH (cCH) do not.
Experts have stated that many people with CH do not get enough pain relief with current treatment options, which are often limited by side effects and contraindications.
GammaCore (electroCore) is a handheld, patient-controlled, non-invasive vagus nerve stimulator used for treating and preventing CH. Patients require only brief training in its use, and it is small, portable and designed to be used anywhere that is convenient. The aim of treatment is to modify pain signals by stimulating the vagus nerve through the skin of the neck. GammaCore can be used acutely when the person feels a CH beginning or daily to help prevent CH.
1.2 Decision Problem (Scope)
In their evidence submission, the company was required to keep within the scope of the evaluation or provide a rationale for any variance. The scope was defined by NICE in the form of a PICO table (population, intervention, comparator, outcomes) plus cost analysis and subgroups to be considered.
1.3 Population
The population of interest was defined as people over the age of 18 years with CH for whom standard of care (SoC) is ineffective or contraindicated. Both eCH and cCH subtypes were included in the scope.
1.4 Intervention
The intervention was defined as gammaCore and gammaCore Sapphire. gammaCore is the original device, which comes pre-loaded with a set number of uses; the whole unit needs to be replaced when finished. gammaCore Sapphire is an upgraded model that can be reloaded by replacing a card in the device and can be recharged using a mains plug. The technology is referred to as gammaCore throughout this article.
1.5 Comparator
The comparator was defined as current SoC for CH and included subcutaneous or nasal spray triptan therapy, oxygen therapy (at home) used alone or alongside subcutaneous or nasal spray triptan therapy, and verapamil, sphenopalatine ganglion (SPG) nerve stimulators or occipital nerve block.
1.6 Outcomes
The following outcomes were included in the scope: frequency, severity and duration of acute episodes of CH, time taken to relieve pain of acute episode (acute use), average response rate and proportion of patients at 50% and 75% response rates, number of times device used for daily prevention, number of times device used for acute treatment, patient-reported pain and disability scores, patient health-related quality of life (including impact on occupation and employment), patient satisfaction, reduction of electrocardiogram and blood testing for monitoring of drug treatments, use of outpatient and healthcare services (including psychiatric care) and device-related adverse events.
A number of subgroup analyses were defined in the scope, including acute treatment of CH and prevention of CH, eCH and cCH.
2 Cedar’s Review of the Evidence
The company provided an evidence submission to NICE that included the available clinical and cost evidence alongside a de novo cost model produced by the company. Cedar’s assessment report aimed to provide the Medical Technologies Advisory Committee (MTAC) with a balanced and independent appraisal of the evidence surrounding the use of gammaCore for CH.
2.1 Review of Clinical-Effectiveness Evidence
The company presented six published studies and two conference abstracts as relevant to the decision problem. Cedar agreed with the inclusion of the selected publications and did not identify any additional published studies for inclusion. The published studies comprised three randomised trials, one post hoc analysis of one of these trials [4,5,6,7], and three non-comparative cohort studies [8,9,10]. The two conference abstracts described a pooled analysis [11] and a post hoc analysis of a randomised trial [12]. GRADE (Grading of Recommendations, Assessment, Development and Evaluation) [13] and CASP (Critical Appraisal Skills Programme) [14] were used to rate the certainty and quality of the evidence. Table 1 provides a summary of the outcomes reported in the included studies.
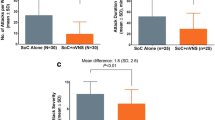
Two trials [5, 6] were European trials and included UK patients, and all three of the cohort studies [8,9,10] were based in the UK. Patient numbers ranged from 25 patients in one study [9] to 150 patients [4], with the three cohort studies having the lowest numbers of patients. Cedar acknowledged that, as the prevalence of CH is very low, large randomised trials would be unlikely. Cedar noted the possibility of an overlap in the patient populations between two of the UK-based studies [9, 10]. All but one of the published studies [8] had company involvement in terms of data collection, analysis and authorship. It is important to note that, in all three trials [4,5,6,7], gammaCore was used in addition to SoC and not in treatment-refractory patients, the population defined in the scope. There is some evidence from two randomised trials [4, 5] that patients with eCH achieved a better response than patients with cCH. However, the trials were not powered for this subgroup analysis, so these results should be considered with caution, particularly as, when considering the whole cohort (eCH and cCH), the benefit of gammaCore was not significant. In addition, a trial [6, 7] that only included patients with cCH reported a significant benefit from using gammaCore.
Overall, the published evidence suggested that patients with CH may benefit from using gammaCore, but the degree of benefit is not clear. As none of the studies had durations of follow-up of more than a few weeks, no evidence of whether any benefit is sustainable long term is available.
2.2 Safety Outcomes
Reported adverse events were mild to moderate in all studies, and no participants discontinued gammaCore use because of adverse events. The most common adverse events related to device use were localised skin tingling or irritation, burning, muscle soreness and/or redness at application site.
Clinical expert comments suggested that gammaCore would be safe and easy for patients with CH to use and that there were very few side effects from using the device; one clinical expert suggested there was no need for safety monitoring.
2.3 Review of Economic Evidence
A systematic review of the literature identified five economic studies; however, none were directly relevant to the scope. Three cost-effectiveness models were for patients with cCH [15,16,17]. gammaCore was the intervention in two of these, set in the USA [15] and Germany [16]. One modelled SPG stimulation in Germany [17]. All models compared costs with those of the acute use of SoC, which comprised triptans and/or oxygen. Two additional models for the cost effectiveness of gammaCore considered a slightly different population in the USA [15] and in the UK [18]. All economic studies were excluded as direct evidence because they were either not set in the UK or were for a different population. They are described in Cedar’s assessment report to provide context and validation for the model [2].
2.3.1 gammaCore Model Structure
The structure was a Markov model with a 1-month cycle, which is an appropriate length. The states included in the model were ‘responder’ and ‘non-responder’, with responder being defined as having at least a 50% reduction in the number of attacks during the assessment period. The model perspective was that of the UK NHS and personal services over a 1-year time horizon with no discounting applied.
The model was for patients with cCH only. It did not include patients with eCH. Patients in each arm were classed as responders and non-responders.
Although the structure was a Markov model, in the base case, patients only moved once, at the end of the first month, and then remained in that state for the remainder of the model, as shown in Table 2. For the first 3 months, in the intervention arm, all patients received gammaCore as a free trial and used it prophylactically. The group of patients classed as “non-responders” experienced some effect during these 3 months and had reduced medication use compared with SoC. After 3 months, they no longer received gammaCore, and their outcomes reverted to those of the SoC group. The resource use groups and resulting costs are also shown in Table 2.
2.3.2 Key Assumptions
The key assumptions in the accepted base model were as follows:
-
Response rates to gammaCore in the PREVA study [6] are generalisable to those of patients eligible for gammaCore in the NHS.
-
In the base case, treatment response is defined as ≥ 50% reduction from baseline in the number of CH attacks per week.
-
Non-responders in the gammaCore plus SoC group received some benefit and reduced medication use during the first 3 months.
-
Non-responders in the gammaCore plus SoC group were assumed to discontinue prophylactic treatment with gammaCore after the 3-month evaluation period but continue use of abortive treatments.
-
For the first month, 8% of the SoC arm had the same rate of medication use as the gammaCore responders. This was a conservative assumption from the manufacturer submission.
-
Beyond 1 month, the rate of medication use for all of the SoC group was taken from the SoC group in the PREVA study [6].
-
Patients were grouped into responder or non-responder in the first month and remained in those groups for the remainder of the model.
-
Non-responders in the gammaCore arm did not receive gammaCore after the third month.
-
Use of abortive medication (conditional on responder status) was assumed to remain constant over time.
2.3.3 Data Sources for Outcomes and Resources
Clinical outcomes were the percentage of patients classed as responders and non-responders, based on at least a 50% reduction in frequency of attacks, from the PREVA trial [6]. Resource use is based entirely on providing gammaCore and the included abortive medications as recorded in the last 14 days of the PREVA trial [6]. Resource use in the company submission for the gammaCore arm (responders) was taken from 35 patients in the PREVA trial [6] who had matched data (attack frequency and resource use) available from both the randomised phase and the open-label phase of the PREVA study [6], whereas the SoC data were taken from a set of 42 patients from a total of 48 intent-to-treat (ITT) population in the randomised phase (Table 3). This was used for both the SoC arm and all but the first 3 months of the gammaCore non-responder arm. None of these data were included in the published PREVA papers [6].
The proportion of patients taking nasal versus subcutaneous sumatriptan was taken from unpublished patient-level data from Marin et al. [10]. No resource uses have been modelled for inpatient, outpatient or general practitioner resources associated with attacks or for any psychological support required to cope with the results of unresponsive cCH. These would be expected to be conservative assumptions since gammaCore is modelled as improving outcomes (shown as a reduction in medication use).
No costs or resources were included for adverse events, and no adverse events associated with cCH were modelled.
gammaCore was provided at no cost for the initial 3-month trial. After this time, patients were required to purchase a card every 3 months to allow the device to function.
2.3.4 Changes by Cedar
Although some uncertainties about the data used and the appropriateness of the patient population existed, Cedar did not identify an alternative more robust data source that could be used in this patient population in this setting. Therefore, no changes were made to the base-case submission, but Cedar did add additional fields to the sensitivity analysis and scenarios.
2.3.5 Results from the Model
The model submitted by the company found that gammaCore saved £450.42 per patient, with sensitivity analysis indicating a highest cost-saving estimate of £1120 per patient and a lowest estimate of − £103 cost incurred per patient.
2.3.6 One-Way Sensitivity Analysis, Scenarios and Key Drivers
The key drivers of the model were the free 3-month trial at the start of gammaCore treatment and the reduced use of sumatriptan.
The company provided additional scenarios based on the categorisation of “responder” at different levels from a 25–65% reduction in frequency of attacks. These scenarios were based on post hoc subgroup analysis of 35 patients in the PREVA trial [6]. One of the main drivers was the use of sumatriptan, and this remained relatively constant (between two and three doses per 14 days) across all the scenarios. The results indicated that use of gammaCore resulted in £343–512 cost savings per patient. An additional scenario used the mean resource use across the whole of the gammaCore arm, as previously presented in Morris et al. [16]. This resulted in cost savings of £104 per patient at 1 year, with all the cost savings occurring during the free trial period and subsequent months being slightly cost incurring.
Cedar created an additional scenario to remove the free 3-month trial from the model and applied this change to all the company scenarios. This resulted in gammaCore becoming cost incurring in the base case and in all scenarios with alternative responder definitions.
3 National Institute for Health and Care Excellence Guidance
3.1 Development of Guidance
The NICE MTAC met in June 2019 and considered evidence from a range of sources, including the company’s submission, Cedar’s report and testimony from clinical experts. The committee made provisional recommendations that went to public consultation.
3.2 Consultation
During the consultation process, NICE received 21 comments from five consultees (four NHS professionals and the company). Comments covered issues including patient response, new evidence, draft recommendations and wording changes. Cedar reviewed the information provided in the comments and provided additional advice to NICE. The comments were discussed at a second MTAC meeting in September 2019.
3.3 Recommendations
Following a period of public consultation and a second committee meeting to discuss responses to consultation, MTAC produced the following recommendations [3]:
-
1.
Evidence supports the case for adopting gammaCore to treat CH in the NHS. gammaCore reduces the frequency and intensity of CH attacks and improves quality of life.
-
2.
gammaCore is not effective in everyone with CH. Treatment with gammaCore should only continue for people whose symptoms reduce in the first 3 months.
-
3.
Cost modelling estimates that, in the first year of treatment, adding gammaCore to SoC is cost saving compared with SoC alone by an average of £450 per person. This cost saving
-
o
Assumes that the first 3-month period of gammaCore use is offered by the company free of charge and
-
o
Largely results from reduced use of subcutaneous sumatriptan.
4 Key Challenges and Learning Points
The evidence relating to CH comprised a small number of studies, with UK-specific evidence limited to observational data. The prevalence of CH in the UK is very low, so a large blinded randomised trial would be difficult to achieve. Overall, the evidence indicates that patients with CH may benefit from using gammaCore in the short term; however, the degree of benefit is not clear, and no evidence exists about whether any benefit is sustainable long term. Subgroup analysis, separating eCH and cCH [11], suggested that patients with eCH achieved better outcomes with gammaCore than with sham treatment. Cedar noted that this result appeared to be driven by the data from the ACT1 trial, which had a much higher proportion of patients with eCH than did the ACT2 trial (n = 101 and n = 30, respectively), and neither trial was powered for subgroup analysis [4, 5]. The result also contrasted with that of the PREVA trial [6], which included only patients with cCH and reported a significant improvement.
A key point to note is that in all three trials, gammaCore was used alongside SoC and not in treatment-refractory patients; therefore, it is possible that the true benefit to patients with CH lies in the addition of gammaCore to their current treatment.
5 Conclusions
Some patients may benefit from using gammaCore as a prophylactic and/or acute treatment for CH. The extent of the benefit is less clear at this time, in terms of both the degree and the duration of response.
It is possible that gammaCore will lead to cost savings; however, this is highly dependent on the availability of the free 3-month trial provided by the company and reductions in use of other medications, primarily sumatriptan.
References
Campbell B, Campbell M. NICE medical technologies guidance: a novel and rigorous methodology to address a new health technology assessment challenge. Appl Health Econ Health Policy. 2012;10(5):295–7.
O’Connell S, Dale M, Morgan H, Carolan-Rees G. MT323 GammaCore for Cluster Headache (external assessment report). 2019. https://www.nice.org.uk/guidance/mtg46/documents/supporting-documentation. Accessed 12 Nov 2020.
National Institute for Health and Care Excellence. gammaCore for cluster headache [MTG46]. National Institute for Health and Care Excellence. 2019. https://www.nice.org.uk/guidance/mtg46. Accessed 12 Nov 2020.
Silberstein SD, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317–32.
Goadsby PJ, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–69.
Gaul C, et al. Non-invasive vagus nerve stimulation for PREVention and acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 2016;36(6):534–46.
Gaul C, et al. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study. J Headache Pain. 2017;18(1):22.
Trimboli M, et al. Non-invasive vagus nerve stimulation for the management of refractory primary chronic headaches: a real-world experience. Cephalalgia. 2018;38(7):1276–85.
Nesbitt AD, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84(12):1249–53.
Marin J, et al. Non-invasive vagus nerve stimulation for treatment of cluster headache: early UK clinical experience. J Headache Pain. 2018;19(1):114.
DeCoo IF, et al. Differential efficacy of non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a meta-analysis. Cephalagia. 2019;39(8):967–77. https://doi.org/10.1177/0333102419856607.
Gaul C et al. Post hoc analysis of the effects of more frequent non-invasive vagus nerve stimulation (nVNS) for patients with chronic cluster headache from the PREVA trial. J Headache Pain. Conference: 12th European Headache Federation Congress and the 32nd National Congress of the Italian Society for the Study of Headaches. Italy. 2018;19(Supplement 1).
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Mwamburi M, et al. Cost-effectiveness of gammaCore (non-invasive vagus nerve stimulation) for acute treatment of episodic cluster headache. Am J Manag Care. 2017;23(16 Suppl):S300–6.
Morris J, et al. Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache. J Headache Pain. 2016;17:43.
Pietzsch J, et al. Cost-effectiveness of stimulation of the sphenopalatine ganglion (SPG) for the treatment of chronic cluster headache: a model-based analysis based on the pathway CH-1 study. J Headache Pain. 2015;16:48.
Jenks M, et al. A preliminary cost-utility analysis of non-invasive vagus nerve stimulation therapy in patients suffering with headache and functional disorder multi-morbidity. Value Health. 2016;19(7):A698.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article was not externally peer reviewed by PharmacoEconomics – Open. This article was reviewed by NICE.
Funding
Cedar was funded by the NICE Medical Technologies Evaluation Programme for their work.
Conflict of interest
Susan O’Connell, Megan Dale and Rhys Morris are employees of the NHS, and Grace Carolan-Rees was an employee of the NHS until September 2020, which has a financial interest in the guidance on which this project is based. Helen Morgan is a Cardiff University employee and has no conflicts of interest that are directly relevant to the content of this article. Kimberley Carter is a NICE employee and had no role in the production of the assessment report but contributed to the preparation of this manuscript. This summary of the MTG was produced following publication of the final guidance report.
Ethics approval, Consent to participate, Consent for publication, Availability of data and materials, Code availability
Not Applicable.
Author Contributions
SOC, MD, HM, RM, GCR and KC contributed to the preparation of this manuscript. GCR reviewed the full assessment report as well as this article. RM reviewed the article and can act as a guarantor for the overall content.
Additional information
GCR was Cedar director until 2020 and has now retired.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
O’Connell, S., Dale, M., Morgan, H. et al. gammaCore for Cluster Headaches: A NICE Medical Technologies Guidance. PharmacoEconomics Open 5, 577–586 (2021). https://doi.org/10.1007/s41669-021-00276-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00276-5



