Abstract
The Centre for Reviews and Dissemination and Centre for Health Economics Technology Assessment Group at the University of York was commissioned by the National Institute for Health and Care Excellence (NICE) Highly Specialised Technologies (HST) programme to act as the independent Evidence Review Group (ERG) for an appraisal of Strimvelis®, a gene therapy treatment for adenosine deaminase deficiency–severe combined immunodeficiency (ADA-SCID). This paper describes the manufacturing company’s submission of clinical and economic evidence, the ERG’s review and the resulting NICE guidance. For Strimvelis® compared with haematopoietic stem cell transplant (HSCT) from a matched unrelated donor (MUD) and HSCT from a haploidentical donor, the company base-case deterministic incremental cost-effectiveness ratios (ICERs) were £36,360 and £14,645 per quality-adjusted life-year (QALY) gained, respectively (using a discount rate of 1.5%). Although overall survival in patients receiving Strimvelis® was substantially higher than historical comparator data on HSCT from a MUD or haploidentical donor, the ERG was concerned that the estimated treatment benefit remained highly uncertain. The ERG critiqued some assumptions in the cost-effectiveness model, including that all patients return to general population mortality and morbidity after a successful procedure; that all patients receive a matched sibling donor following an unsuccessful engraftment; and that differences in wait times exist between the treatments. Incorporating a number of changes to the model, the ERG’s base-case ICERs were £86,815 per QALY gained for Strimvelis® compared with HSCT from a MUD and £16,704 per QALY gained compared with HSCT from a haploidentical donor (using a discount rate of 1.5%). The ICER for Strimvelis® compared with HSCT from a MUD was highly sensitive to the difference in procedural mortality and could exceed NICE’s £100,000 per QALY gained threshold for HSTs, if HSCT survival rates have improved since the most recent data. The evaluation committee concluded that the most plausible ICERs were lower than £100,000 per QALY gained and that Strimvelis® should be recommended for treatment of ADA-SCID where a matched related donor is unavailable.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
The funding arrangements for Strimvelis® were unknown at the time of appraisal and its cost was expected to fluctuate with the exchange rate. |
The treatment benefit with Strimvelis® was uncertain and the incremental cost-effectiveness ratio (ICER) for Strimvelis® compared with haematopoietic stem cell transplant from a matched unrelated donor was very sensitive to the assumed difference in procedural mortality between the treatments. |
The Highly Specialised Technologies Evaluation Committee concluded that the most plausible ICERs were under £100,000 per quality-adjusted life-year gained and recommended Strimvelis® for the treatment of adenosine deaminase deficiency–severe combined immunodeficiency where a matched related donor is unavailable. |
1 Introduction
The National Institute for Health and Care Excellence (NICE) is an independent body that produces guidance and advice on health and social care in England. The Highly Specialised Technologies (HST) programme evaluates highly specialised treatments for very rare conditions. As part of the process, an Evidence Review Group (ERG) independently reviews clinical and economic evidence that is submitted by the company manufacturing the technology. It presents its critique for an independent Evaluation Committee which considers it alongside other evidence and makes recommendations to NICE. Given the very small numbers of patients with the very rare conditions involved, the HST committee does not employ a simple utilitarian approach but must still give consideration to the balance between costs and benefits. Below a most plausible incremental cost-effectiveness ratio (ICER) of £100,000 per quality-adjusted life-year (QALY) gained, the decision to recommend the use of an HST is normally based on the cost-effectiveness estimate. Above a most plausible ICER of £100,000 per QALY gained, judgements about the acceptability of the HST as an effective use of National Health Service (NHS) resources must take account of the magnitude of the incremental therapeutic improvement, as revealed through the number of additional QALYs gained [1].
This article summarises the ERG’s critique of the manufacturing company’s submission on Strimvelis® (retroviral-transduced autologous CD34+ cells) for treating adenosine deaminase deficiency–severe combined immunodeficiency (ADA-SCID). It also summarises issues that arose during the review and the committee’s decision-making. Full details of the appraisal and documents can be found on the NICE website [2].
2 The Decision Problem
ADA-SCID is a rare, inherited immune disorder, characterised by profound lymphopenia and impaired development and function of T cells, B cells and natural killer cells, as well as non-immunological defects [3]. Patients present with severe infections and failure to thrive [3] and are usually diagnosed within the first year of life [4]. If infants with ADA-SCID receive no treatment to restore immune function, they will likely die before the age of 2 years [4].
There are very limited data available to estimate the incidence of ADA-SCID in England, which is likely to be very low and concentrated within certain ethnicities in the UK [5]. Based on an estimate of 20 children per year presenting in the UK with any form of SCID [6], the company estimated that there would be three or fewer patients a year diagnosed with ADA-SCID in England.
There is no NICE clinical guidance on the management of ADA-SCID, although there are treatment guidelines from the European Society for Blood and Marrow Transplantation (EBMT) and European Society for Immunodeficiencies (ESID) [7]. The current preferred treatment is haematopoietic stem cell transplant (HSCT) from a human leukocyte antigen (HLA) matched related donor (MRD) [7, 8]. Survival rates for ADA-SCID patients undergoing HSCT from an MRD were 86% for matched sibling donors (MSDs) and 83% for matched family donors (MFDs) in the most recent published data at the time of the appraisal [3].
If no MRD is available, treatment options include gene therapy, HSCT from a matched unrelated donor (MUD), HSCT from a haploidentical donor and enzyme replacement therapy (ERT) with polyethylene glycol-modified bovine adenosine deaminase (PEG-ADA) [7, 8]. The EBMT/ESID guidelines recommend gene therapy in these cases [7] and there are ongoing trials of gene therapy treatments [8]. Strimvelis® is a gene therapy treatment licensed for use in patients with ADA-SCID for whom no MRD is available, and this was the patient population considered in the submission. It was given European Union (EU) marketing authorisation in May 2016 and was the first EU-approved ex vivo gene therapy for paediatric patients. Cells from the patient’s own bone marrow are transduced to express ADA and, provided engraftment is successful, it is designed to be a single treatment with lifelong effects. Strimvelis® is currently only available at the Hospital San Raffaele Telethon Institute for Gene Therapy in Milan, Italy. Treatment is only possible if patients can donate sufficient CD34+ cells to deliver a minimum of 4 million purified CD34+ cells/kg for the manufacture of Strimvelis® and a CD34+ stem cell back-up for use as a rescue treatment. In the clinical pathway presented, patients are initially maintained on intravenous immunoglobulin (IVIG) after treatment with Strimvelis® or HSCT, and eventually discontinue IVIG if treatment is successful.
According to the submission, Strimvelis® requires less pre-conditioning than HSCT from a MUD or haploidentical donor, does not carry the same risk of graft versus host disease (GvHD) and does not require the search for a donor that can delay HSCT from a MUD. GvHD is a complication associated with all types of allogenic HSCT. It is an immunological disorder that can be acute or chronic, and can affect many organs and cause death [9]. As gene therapy involves the patient’s own stem cells (autologous), the risk of GvHD is eliminated [10].
The comparator identified by NICE was HSCT from a MUD or haploidentical donor. In the most recent published data on ADA-SCID patients, survival rates were 67% for HSCT from a MUD (1995–2009) and 71% for HSCT from a haploidentical donor (2000–2009) [3]. These treatments have a high morbidity and mortality risk associated with GvHD [3, 11]. HSCT from a MUD or haploidentical donor (but not normally MRD) usually requires chemotherapeutic pre-conditioning, although there is no consensus on conditioning regimens [10, 12]. According to the submission, the low-dose busulfan conditioning used before treatment with Strimvelis® would be associated with fewer adverse events than the full-dose chemotherapy regimens often used for HSCT from a MUD or haploidentical donor.
ERT with PEG-ADA is a non-curative and expensive treatment [8] which requires frequent injections and regular monitoring [10]. There is some uncertainty over long-term efficacy, as a study of nine patients receiving PEG-ADA for 5–12 years found that patients had subnormal immune function [13]. It is not approved in the EU and according to expert clinical advice is used only as a bridge to curative treatment in UK clinical practice, with patients initiated on PEG-ADA immediately following diagnosis of ADA-SCID while awaiting HSCT or gene therapy.
3 The Independent Evidence Review Group (ERG) Review
The company submitted evidence to NICE on the use of Strimvelis® in patients with ADA-SCID. In accordance with HST procedures, the ERG had the opportunity to seek clarification on specific issues in the submission and request additional information from the company. The ERG reviewed and critiqued this submission and checked for the existence of other evidence or alternative interpretations of the evidence.
3.1 Clinical Evidence
The company’s submission of evidence included a systematic review of studies on the use of Strimvelis® and of the comparator treatments. Four open-label, single-arm trials of Strimvelis® were identified, as well as a long-term follow-up (LTFU) study of patients from these trials [14]. The sample sizes of the trials were very small, ranging from one to 12 patients (with 17 of the 18 total patients enrolled in the LTFU). The evidence submitted by the company focused on an integrated population of the 18 patients that had received Strimvelis® as part of these studies, with data pooled and treated as if it were from a single study. Data on some further patients receiving Strimvelis® as part of a Named Patient Programme (NPP) were also provided but not included in the evidence synthesis or base-case cost-effectiveness analysis. A historical comparator was used, with data on HSCT from a MUD or haploidentical donor in ADA-SCID patients based primarily on a multicentre, retrospective study, which provided the most recent survival data [3]. Some smaller case series and reports were also included in the narrative synthesis.
Overall survival in all 18 patients in the Strimvelis® integrated population was 100%, with a median follow up of 6.95 years (range 2.3–13.4 years). Intervention-free survival was achieved in 14 of 17 patients (82.3%). Of the three patients in whom Strimvelis® was unsuccessful, two underwent HSCT from an MSD and one received a second dose of Strimvelis® and long-term PEG-ADA treatment.
Based on the main comparator study [3], overall survival for those receiving HSCT from a MUD was 67% (10/15) between 1995 and 2009. For HSCT from a haploidentical donor, overall survival was 71% (5/7) between 2000 and 2009. Earlier data on HSCT from a haploidentical donor were not used in the comparison due to improvements in effectiveness of HSCT over time. Intervention-free survival data were limited for the comparator treatments, and it was not clear if available data were comparable with the data on Strimvelis®.
Adverse events were similar for Strimvelis® and the comparator treatments. All but one patient in the Strimvelis® integrated population experienced a neurological, central nervous system or hearing event during treatment or follow-up. A high incidence of non-immunological problems was also found in ADA-SCID patients following HSCT [15,16,17,18,19]. The company concluded that neither treatment appears to be effective in reducing non-immunological problems. The main difference in adverse events was that some patients experienced GvHD after HSCT [15, 17, 19,20,21,22,23,24], whereas no patients experienced this following treatment with Strimvelis®.
Data were also presented on other outcomes, including immune function. Despite some variability across outcomes and over time, generally data showed improved immune function with Strimvelis®. For example, the severe infection rate declined, CD3+ T cell counts increased, and most patients that discontinued IVIG exhibited antibodies to a number of infectious antigens. There was a lack of comparable data after HSCT from a MUD or haploidentical donor but both comparator treatments also appeared to improve immune function [3].
3.2 Critique of Clinical Evidence
The ERG highlighted a number of issues with the clinical evidence presented.
3.2.1 Study Design
The clinical evidence presented was based on open-label, single-arm trials with very small patient numbers and a historical comparator. Studies and comparisons of this nature are inherently at high risk of bias and lack precision in estimation of effects. Although the ERG considered this study design appropriate to evaluate clinical effectiveness due to the low incidence of ADA-SCID, there remained important limitations.
3.2.2 Evidence Synthesis
The ERG considered there to be sufficient similarity between the studies that made up the Strimvelis® integrated population that pooling the data and treating them as if comprising a single study was unlikely to lead to substantial bias. However, the ERG did not consider it appropriate to exclude data from the NPP from the narrative synthesis of clinical evidence, given the small sample size of the integrated and total population and the need to consider all available data.
3.2.3 Differences with the Eligible Population
There were some concerns regarding how representative the Strimvelis® integrated population was of ADA-SCID patients in England. There was a lack of clarity regarding numbers screened or excluded for some of the studies, making it unclear if patients at greater risk had been excluded or whether other selection biases were present. No patients had been confirmed as having active viral infection at screening. Given the potential for viral infection in ADA-SCID patients, and expert clinical advice to the ERG that viral infection may be prognostic for success of treatment [11, 25], the extent to which the data could be generalised to patients presenting with viral infections is unclear. The duration of PEG-ADA use by patients that received Strimvelis® was longer than would be expected in UK practice, although there is no evidence that this is prognostic for success of subsequent treatment. Whilst noting these concerns, overall the ERG concluded that the data on Strimvelis® were likely to be generalisable to England.
3.2.4 Survival Rates
Although overall survival was higher for Strimvelis® than for the comparator treatments, the ERG believed there to be significant limitations to the data. Firstly, this evidence was based on small patient numbers so a small number of deaths could lead to substantial changes in survival estimates. Secondly, the historical data on overall survival following HSCT likely reflect an underestimate of the current effectiveness of these treatments, due to the small sample sizes, new techniques in allogeneic HSCT [8] and improved survival rates in more recent data on HSCT in patients with other conditions [26, 27]. There have been improvements in matching of donors, infection control procedures, intensity of conditioning and provision of supportive care [27]. Thirdly, the overall survival rate overestimated the effectiveness of Strimvelis®, since when treatment failed but patients did not die due to receiving an alternative treatment, they were still counted as a treatment success. In the view of the ERG, the intervention-free survival rate provided a better assessment of clinical effectiveness, as also noted by the European Medicines Agency [28].
3.2.5 Adverse Events
There were limitations in the reporting of GvHD following HSCT. Estimates were based on very small case reports (ranging from one to seven patients) and definitions and reporting of GvHD in these studies varied. Data from studies from different centres and time periods were pooled as if from a single study rather than using meta-analytic methods. Regarding Strimvelis®, leukaemia-like lymphoproliferative disorders have been identified in patients with other forms of SCID after gene therapy due to the vector being integrated near an oncogene [29, 30]. Although no similar events have been observed in ADA-SCID, the small numbers of patients who have received Strimvelis® mean this cannot yet be ruled out as an important potential risk.
3.3 Cost-Effectiveness Evidence
The cost-effectiveness evidence provided by the company included a review of published data on health-related quality of life (HRQoL) and a de novo economic evaluation, comparing Strimvelis® to either HSCT from a MUD or haploidentical donor in a hypothetical cohort of patients aged 1 year old. The model consisted of a decision tree to establish the proportion of patients surviving the initial transplant procedure and the proportion requiring rescue transplant in the first 3 years, combined with a Markov modelling approach to extrapolate costs and quality-adjusted survival over a lifetime time horizon. The four main outcomes from the model were (1) success, long-term survival; (2) unsuccessful engraftment; (3) death; and (4) long-term survival after rescue HSCT.
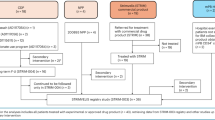
The model assumed patients would receive PEG-ADA treatment while awaiting initial or rescue transplant procedures, and incorporated post-procedure IVIG use and risk of severe infection for any transplant procedure and GvHD for HSCT. Rescue transplants were assumed to be HSCT from an MSD (e.g. a new sibling born since the decision to undergo treatment with Strimvelis®) and to occur 2 years after the initial procedure, with no risks of death, GvHD or failure to engraft. It was assumed that the decision to use Strimvelis® would be made before the search for a MUD, and that HSCT from a haploidentical donor would only be used if a MUD was unavailable. Patients who survived transplant procedures were assumed to return to the mortality and morbidity risk of the general population. The company’s model structure is presented in Fig. 1.
Schematic of the company’s model structure. ADA-SCID adenosine deaminase deficiency–severe combined immunodeficiency, GvHD graft versus host disease, HSCT haematopoietic stem cell transplant, IVIG intravenous Immunoglobulin, MRD matched related donor, MSD matched sibling donor, PEG-ADA polyethylene glycol-modified bovine adenosine deaminase
The model characterised three main benefits of treatment with Strimvelis®: (1) reduced duration of ERT with PEG-ADA before the initial transplant procedure; (2) reduced procedural mortality; and (3) avoidance of GvHD. The model also assumed different rates of rescue transplant between treatment arms.
The company model assumed overall survival of 100% with Strimvelis®, 66.67% with HSCT from a MUD and 71.4% with HSCT from a haploidentical donor, based on data from the Strimvelis® integrated population and Hassan et al. [3] as the historical comparator. Rates of rescue transplant following failure to engraft were assumed to be 17.6, 6.7 and 28.6%, respectively. GvHD was assumed to occur in approximately one-third of patients undergoing an initial HSCT procedure, while there was assumed to be no risk of GvHD with Strimvelis®. HRQoL was assumed equal to that of the general population, with decrements applied for 6 months in patients recovering from transplant procedures and in patients experiencing GvHD. The perspective of the company’s analysis was the NHS and Personal Social Services (NHS & PSS).
The company argued that a discount rate of 1.5% should be applied to costs and health outcomes, on the basis that Strimvelis® restores people to full or near full health over a long time period who would have otherwise died or had a very severely impaired life. The company base case found Strimvelis® to be more costly and more effective than the comparators. The deterministic ICERs were £36,360 per QALY gained for Strimvelis® compared with HSCT from a MUD and £14,645 per QALY gained for Strimvelis® compared with HSCT from a haploidentical donor. The ICERs remained below the £100,000 per QALY lower tier threshold that NICE considers appropriate for HSTs [1], across a range of one- and two-way sensitivity analyses and scenario analyses.
3.4 Critique of Cost-Effectiveness Evidence
The ERG highlighted a number of issues with the cost-effectiveness evidence presented by the company.
3.4.1 Position of Strimvelis® in the Treatment Pathway
The ERG was concerned that the model did not include alternative points in the treatment pathway at which a decision to use Strimvelis® may be taken. The company model applied only to patients for whom the decision is taken immediately following diagnosis, thus avoiding a search for a MUD for all patients (where a search includes a database search, followed by contacting and testing potential donors). The ERG considered that for some patients the decision to use Strimvelis® may be taken only after a search for a MUD has been completed, including those unwilling to travel to Milan if alternative treatment is available.
3.4.2 Health Gains with Strimvelis®
The ERG believed that the respective 33 and 29 percentage point reductions in procedural mortality with Strimvelis® compared with HSCT from a MUD or haploidentical donor applied in the submission may represent the upper limit of additional benefit from Strimvelis®. This was due to the likely improvements in survival from HSCT and the use of overall survival rather than intervention-free survival to characterise the efficacy of Strimvelis®.
The ERG thought it was unrealistic to assume that patients with ADA-SCID who survive an initial transplant procedure (either Strimvelis® or HSCT) return to the same level of health and life expectancy as the general population. The ERG felt that this would overestimate quality-adjusted survival and underestimate healthcare costs due to the cognitive and neurological deficits of ADA-SCID, such as bilateral hearing impairment, and potential long-term adverse events associated with pre-transplant conditioning regimens. Of similar concern was the assumption of 100% success and survival with rescue transplant, which overestimates quality-adjusted survival and underestimates the healthcare costs in patients that fail to engraft following the initial procedure. These factors caused the company model to overestimate the health benefit from reductions in procedural mortality and underestimate subsequent costs.
3.4.3 Treatment Costs Associated with Strimvelis® and HSCT
The ERG identified a number of costs associated with Strimvelis® that were omitted from the company base case. These included NHS-supported travel costs to and from Milan, the cost of screening patients deemed unable to produce sufficient CD34+ cells, additional hospitalisation costs for patients whose length of stay exceeds 55 days and transplantation of back-up bone marrow after treatment failure or to facilitate recovery.
The cost per HSCT from a MUD and GvHD event in the company base case appeared to have been overestimated. For HSCT, the company applied a unit cost taken from the NHS main schedule of reference costs specific to cord blood transplants (£95,517). The majority of stem cell transplants are sourced from bone marrow, which was the comparator noted in the scope, and which has a lower average cost in the NHS schedule (£79,199) than cord blood transplants [3, 31]. The unit cost applied to GvHD events of any severity in the model was the mean difference in hospital readmission costs between patients without GvHD and those with severe (grade III/IV) GvHD [32]. The same study provided a cost estimate for any GvHD event, which the ERG deemed more appropriate.
The ERG had concerns about the company’s assumed 10-week differential in wait times between Strimvelis® (9 weeks) and HSCT (19 weeks). The wait time for Strimvelis® was based on the clinic schedule. During clarification the ERG requested information on the observed wait time among patients in the integrated population. The company response indicated that the average wait time was 5.7 months between the decision to use Strimvelis® and receipt of Strimvelis®.
The ERG identified two potentially relevant uncertainties in the product cost of Strimvelis®. First, it was unclear whether value-added tax (VAT) should be added to the fixed price in euros agreed between the company and NHS England. VAT may be payable dependent on whether a patient arrives in Milan via the S2 or EU directive route, and this was not decided at the time of appraisal. Second, the ERG deemed that the uncertainty in the exchange rate warranted consideration.
3.4.4 Preferred Base Case
The ERG made a number of changes to the company model, combining scenario analyses provided by the company with those formed by the ERG, as shown in Table 1.
The ERG’s base case predicted lower QALYs for all three treatments than the company base case. This was due to increased mortality and morbidity associated with rescue transplants and the application of HRQoL decrements for IVIG use and bilateral hearing impairment in patients successfully treated with HSCT or Strimvelis®. It predicted higher costs for Strimvelis® and HSCT from a haploidentical donor and lower costs for HSCT from a MUD. This was attributable to a correction in the calculation of rates of rescue transplant, combined with increased healthcare costs per rescue transplant to reflect risks of severe infection and GvHD, and the lower unit cost for HSCT from a MUD.
The ERG’s base-case ICERs were higher than those estimated by the company, at £86,815 per QALY gained for Strimvelis® compared with HSCT from a MUD and £16,704 per QALY gained compared with a haploidentical donor using a discount rate of 1.5%. Using the NICE reference case 3.5% discount rate, the ICER for Strimvelis® compared with HSCT from a MUD was £147,834 per QALY gained and £28,503 per QALY gained when compared with HSCT from a haploidentical donor. The ICER for Strimvelis® compared with HSCT from a MUD was very sensitive to the assumed difference in procedural mortality between the two treatments. Strimvelis® must reduce procedural mortality by at least 30 percentage points compared with HSCT from a MUD in order for the ICER to remain below £100,000 per QALY gained when using a 1.5% discount rate. The ICER was also sensitive to the rates of rescue transplants and the additional cost of Strimvelis®. The ICERs for Strimvelis® compared with HSCT from a MUD or haploidentical donor increased if search costs for a MUD are not avoided prior to Strimvelis® treatment. It was anticipated that the ICERs for Strimvelis® compared with HSCT from a MUD or haploidentical donor may increase in patients that have a worse prognosis, including older patients and patients with active viral infection.
3.5 Conclusions of the ERG’s Review
Overall survival was 100% for Strimvelis®, substantially higher than historical comparator data, and intervention-free survival was 82.3%. However, the ERG was concerned that the extent of the estimated treatment benefit is highly uncertain due to the small patient numbers. The ERG considered that the company base case omitted potentially important costs associated with the use of Strimvelis® and may have overestimated some costs associated with the comparator treatments. The ERG was also concerned by some of the assumptions in the model, in particular that all patients who survive the initial procedure are cured and return to general population mortality and morbidity. The ERG’s preferred base case suggested that Strimvelis® is cost effective, with respect to NICE’s £100,000 lower tier threshold for HSTs, for patients that have no MUD available and in whom HSCT from a haploidentical donor is the only alternative. However, improvements in techniques that increase overall survival after HSCT from a MUD or the occurrence of a death in a patient treated with Strimvelis® could cause the ICER for Strimvelis® compared with HSCT from a MUD to exceed £100,000 per QALY gained.
4 National Institute for Health and Care Excellence (NICE) Guidance
The HST Evaluation Committee considered the ERG report alongside evidence submitted by the manufacturer and the views of parents or carers of patients, patient representatives and clinical experts [33].
The committee considered the approach to the clinical effectiveness evidence taken by the company to be appropriate. Despite uncertainty on overall survival, it was decided that survival rates are higher with Strimvelis® than the comparators and that data on intervention-free survival, while important, cannot easily be compared with HSCT. In terms of differences with the eligible population, the committee concluded that patients in clinical practice may be younger than those in the evidence presented, potentially leading to greater efficacy.
The committee disagreed with the ERG on the position of Strimvelis® in the treatment pathway, after hearing from clinical experts that they would perform a database search for a MUD (but not contact and test donors) before the decision to use Strimvelis® is made. A database search is quick, with the main costs and wait time of finding a MUD incurred during contacting and testing potential donors. The committee also heard from experts that it would not be possible to receive Strimvelis® as a rescue treatment after failure of HSCT and so concluded that the pathway used by the company was appropriate.
The committee agreed with the company that it was appropriate to exclude data from the NPP from the economic model due to differences with the population likely to receive Strimvelis® in clinical practice, and that costs associated with bilateral hearing impairment should not be included. It also decided that a higher survival rate from HSCT with a MUD should be used to reflect the uncertainty and likely underestimate of the historical comparator data [3], and agreed on a value of 72.5%. Table 2 presents the associated costs, effects and cost effectiveness in the committee’s preferred model.
The committee had concerns about whether Strimvelis® fulfilled NICE’s criteria for using a 1.5% discount rate [1] and considered both a 1.5% and 3.5% rate. The analysis using the committee’s preferred assumptions with a 1.5% discount rate gave ICERs of £74,430 per QALY gained for Strimvelis® compared with HSCT from a MUD and Strimvelis® was dominant when compared with HSCT from a haploidentical donor. Using a 3.5% discount rate, the ICER for Strimvelis® compared with HSCT from a MUD was £120,506 per QALY gained and £12,106 per QALY gained for Strimvelis® compared with HSCT from a haploidentical donor. The committee also considered a scenario in which rescue rates were equal between treatments; the ICERs for Strimvelis® compared with HSCT from a MUD were lower in this scenario and the ICERs for Strimvelis® compared with a haploidentical donor remained under £100,000 per QALY gained. As the committee believed Strimvelis® offers undiscounted QALY gains of at least 14.0 compared with HSCT from a MUD, they applied a QALY weighting of 1.4 to this comparison, in accordance with the HST programme process [1].
The committee believed that the most plausible ICERs for Strimvelis® compared with HSCT from a MUD or haploidentical donor were lower than the £100,000 per QALY gained threshold. While acknowledging the high cost of Strimvelis® and some uncertainty in both the clinical and cost-effectiveness evidence provided, the committee considered Strimvelis® to be an effective treatment that provides value for money.
After consultation on preliminary guidance, NICE issued the following final guidance on the use of Strimvelis®:
“Strimvelis is recommended, within its marketing authorisation, as an option for treating adenosine deaminase deficiency–severe combined immunodeficiency (ADA–SCID) when no suitable human leukocyte antigen-matched related stem cell donor is available” [34].
5 ERG Conclusion
This HST evaluation highlighted the importance of recommendations being informed by an independent ERG critique of the evidence. In this case, the ERG identified several important limitations to the data presented and was able to critique the economic model and provide additional analyses using the best available evidence.
There were a number of general issues arising from the HST evaluation which may be of importance in other cases, including evaluations of other gene therapy treatments. Firstly, it highlighted the challenges of appraising clinical and cost-effectiveness evidence on very rare conditions. Conclusions were highly sensitive to the interpretation of very limited data. Evidence on Strimvelis® relied on single-arm studies with very small patient numbers and historical comparator data. This resulted in a high level of uncertainty both in terms of the inherent risk of bias associated with this study design and a lack of precision due to small sample sizes for both Strimvelis® and historical comparators. Each patient treated could have a large influence on estimates of overall survival and treatment success. Given the rarity of the disease, there were also some issues with the representativeness of the population that had received Strimvelis® to the eligible population in England. While there is a well-developed methodological literature for evaluating randomised controlled trials in much larger patient populations, there is less guidance on assessing study designs most appropriate for evaluating specialised technologies in rare conditions. Therefore, extensive reflection and careful judgement were required to assess whether appropriate methods were used to minimise confounding and other biases.
Secondly, it highlighted the value of up-to-date published data on treatment outcomes for rare conditions, particularly in treatments such as HSCT where outcomes are improving rapidly. Obtaining contemporary evidence to inform survival rates was challenging and an update to the latest analysis would have been very valuable in determining whether Strimvelis® could be considered cost effective. It also emphasised the importance of consulting with experts in the relevant field of medicine to obtain up-to-date survival estimates.
Thirdly, it raised questions over the relative usefulness of overall survival as a key outcome, when relying on small, single-arm trials of this nature. Some patients were counted as a treatment success despite having to receive an alternative treatment, without which they may have died. The ERG considered the intervention-free survival rate to be a better assessment of effectiveness.
Finally, the ERG raised the possibility of Strimvelis® being offered at a different point in the treatment pathway, if the requirement to move temporarily to Milan acted as a barrier to some families. This may be particularly relevant in other cases where treatment is provided in another country. The critique of the cost-effectiveness evidence also raised issues around the costs of travel to Italy and uncertainties around treatment costs due to exchange rates and VAT.
References
National Institute for Health and Care Excellence. Interim process and methods of the Highly Specialised Technologies programme (updated to reflect 2017 changes). NICE; 2017. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-highly-specialised-technologies-guidance. Accessed 13 Jun 2018.
National Institute for Health and Care Excellence. Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency. Highly specialised technologies guidance [HST7]. NICE; 2018. https://www.nice.org.uk/guidance/hst7. Accessed 13 Jun 2018.
Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase–deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–24.
Hershfield M. Adenosine deaminase deficiency. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al., editors. GeneReviews®. Seattle: University of Washington; 2006 (Internet; updated 2017 Mar 16).
Adams SP, Wilson M, Harb E, Fairbanks L, Xu-Bayford J, Brown L, et al. Spectrum of mutations in a cohort of UK patients with ADA deficient SCID: segregation of genotypes with specific ethnicities. Clin Immunol. 2015;161(2):174–9.
Bazian Ltd. Screening for severe combined immunodeficiency: external review against programme appraisal criteria for the UK National Screening Committee (UK NSC). UK National Screening Committee; 2012.
European Society for Blood and Marrow Transplantation, European Society for Immunodeficiencies. EBMT/ESID guidelines for haematopoietic stem cell transplantation for primary immunodeficiencies. European Society for Blood and Marrow Transplantation; 2017.
Kohn DB, Gaspar HB. How we manage adenosine deaminase-deficient severe combined immune deficiency (ADA SCID). J Clin Immunol. 2017;37(4):351–6.
Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61.
Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009;114(17):3524–32.
Pai S-Y, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–46.
Gaspar HB, Qasim W, Davies EG, Rao K, Amrolia PJ, Veys P. How I treat severe combined immunodeficiency. Blood. 2013;122(23):3749.
Chan B, Wara D, Bastian J, Hershfield MS, Bohnsack J, Azen CG, et al. Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol. 2005;117(2):133–43.
Cicalese MP, Ferrua F, Castagnaro L, Pajno R, Barzaghi F, Giannelli S, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. 2016;128(1):45–54.
Booth C, Hershfield M, Notarangelo L, Buckley R, Hoenig M, Mahlaoui N, et al. Management options for adenosine deaminase deficiency; proceedings of the EBMT satellite workshop (Hamburg, March 2006). Clin Immunol. 2007;123(2):139–47.
Rogers MH, Lwin R, Fairbanks L, Gerritsen B, Gaspar HB. Cognitive and behavioral abnormalities in adenosine deaminase deficient severe combined immunodeficiency. J Pediatr. 2001;139(1):44–50.
Honig M, Albert MH, Schulz A, Sparber-Sauer M, Schutz C, Belohradsky B, et al. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109(8):3595–602.
Albuquerque W, Gaspar HB. Bilateral sensorineural deafness in adenosine deaminase-deficient severe combined immunodeficiency. J Pediatr. 2004;144(2):278–80.
Borghans JA, Bredius RG, Hazenberg MD, Roelofs H, Jol-van der Zijde EC, Heidt J, et al. Early determinants of long-term T-cell reconstitution after hematopoietic stem cell transplantation for severe combined immunodeficiency. Blood. 2006;108(2):763.
Bhattacharya A, Slatter MA, Chapman CE, Barge D, Jackson A, Flood TJ, et al. Single centre experience of umbilical cord stem cell transplantation for primary immunodeficiency. Bone Marrow Transpl. 2005;36(4):295–9.
Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295:508–18.
Baffelli R, Notarangelo LD, Imberti L, Hershfield MS, Serana F, Santisteban I, et al. Diagnosis, treatment and long-term follow up of patients with ADA deficiency: a single-center experience. J Clin Immunol. 2015;35:624–37.
Dvorak CC, Hassan A, Slatter MA, Hönig M, Lankester AC, Buckley RH, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134(4):935–43.
Serana F, Sottini A, Chiarini M, Zanotti C, Ghidini C, Lanfranchi A, et al. The different extent of B and T cell immune reconstitution after hematopoietic stem cell transplantation and enzyme replacement therapies in SCID patients with adenosine deaminase deficiency. J Immunol. 2010;185(12):7713–22.
Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ, Veys P, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–10.
UK Stem Cell Strategy Oversight Committee. Unrelated donor stem cell transplantation in the UK: effective affordable sustainable. NHS Blood and Transplant; 2014.
Svenberg P, Remberger M, Uzunel M, Mattsson J, Gustafsson B, Fjaertoft G, et al. Improved overall survival for pediatric patients undergoing allogeneic hematopoietic stem cell transplantation—a comparison of the last two decades. Pediatr Transpl. 2016;20(5):667–74.
European Medicines Agency (EMA). Assessment report for strimvelis, common name: autologous CD34+ enriched cell fraction that contains CD34+ cells transduced with retroviral vector that encodes for the human ADA cDNA sequence. Procedure no. EMEA/H/C/003854/0000. London: EMA; 2016.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;3021:415–9.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–50.
van Agthoven M, Groot MT, Verdonck LF, Lowenberg B, Schattenberg AVMB, Oudshoorn M, et al. Cost analysis of HLA-identical sibling and voluntary unrelated allogeneic bone marrow and peripheral blood stem cell transplantation in adults with acute myelocytic leukaemia or acute lymphoblastic leukaemia. Bone Marrow Transpl. 2002;30(4):243–51.
Dignan FL, Potter MN, Ethell ME, Taylor M, Lewis L, Brennan J, et al. High readmission rates are associated with a significant economic burden and poor outcome in patients with grade III/IV acute GvHD. Clin Transpl. 2013;27(1):E56–63.
National Institute for Health and Care Excellence. Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency. Evaluation consultation document. NICE; 2017. https://nice.org.uk/guidance/hst7/history. Accessed 9 Jan 2018.
National Institute for Health and Care Excellence. Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency. Final evaluation determination. NICE; 2017. https://nice.org.uk/guidance/hst7/history. Accessed 9 Jan 2018.
Acknowledgements
The authors would like to thank Dr. Andrew Gennery, Clinical Reader and Consultant at Great North Children’s Hospital, for clinical advice throughout the project. They would also like to thank Melissa Harden, Centre for Reviews and Dissemination, who critiqued the literature searches in the Company’s submission, and Professor Stephen Palmer, Centre for Health Economics, who provided advice on the cost-effectiveness part of the project.
Author information
Authors and Affiliations
Contributions
Susan Griffin, Edward Cox, Nick Meader, Emily South and Nerys Woolacott all formed part of the ERG that produced the ERG report described in this paper. Susan Griffin and Nick Meader took overall responsibility for the cost and clinical effectiveness parts of the project. Emily South wrote the draft of the manuscript. All authors commented on the manuscript and approved the final version. This article has not been externally peer reviewed by PharmacoEconomics Open.
Corresponding author
Ethics declarations
Funding
This study was funded by National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 16/27/01). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
Conflict of Interest
Emily South, Edward Cox, Nick Meader, Nerys Woolacott and Susan Griffin declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
South, E., Cox, E., Meader, N. et al. Strimvelis® for Treating Severe Combined Immunodeficiency Caused by Adenosine Deaminase Deficiency: An Evidence Review Group Perspective of a NICE Highly Specialised Technology Evaluation. PharmacoEconomics Open 3, 151–161 (2019). https://doi.org/10.1007/s41669-018-0102-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-018-0102-3