Abstract
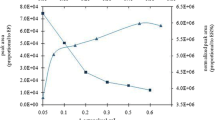
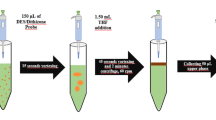
A novel analytical procedure for the determination of gold by electrothermal atomic absorption spectrometry combined with selective liquid–liquid extraction by natural deep eutectic solvents (NADESs) is presented. The extraction ability of the NADESs prepared using menthol, thymol and camphor toward gold in hydrochloric acid solutions was studied. The extraction efficiency was improved by optimizing the parameters including the composition of the NADESs, the volume ratio of organic and aqueous phases, kinetics, and acidity of the solution. Quantitative determination of gold was carried out by electrothermal atomic absorption spectrometry directly in the NADESs phase dissolved in isopropyl alcohol. The extraction recovery of gold from 1 mol/L HCl with the NADESs based on menthol and camphor mixed in a molar ratio 1:1 was 99.7% at an enrichment factor of 100. The limits of detection and quantification of the proposed procedure were 1 μg/L and 3.3 μg/L, respectively, with a relative standard deviation of less than 5%. The developed procedure was applied for determination of gold in the certified reference material of ore, environmental and waste waters.




Similar content being viewed by others
Data availability
The data supporting the finding reported herein are available on reasonable request from the corresponding author.
References
Yakubchuk A. Russian gold mining: 1991 to 2021 and beyond. Ore Geol Rev. 2023;153:105287.
Grebneva-Balyuk ON, Kubrakova IV. Determination of platinum group elements in geological samples by inductively coupled plasma mass spectrometry: possibilities and limitations. J Anal Chem. 2020;75(3):275–85.
Liu YH, Wan B, Xue DS. Sample digestion and combined preconcentration methods for the determination of ultra-low gold levels in rocks. Molecules. 2019;24(9):24091778.
Dubenskiy AS, Bolshov MA, Seregina IF. Sorption–mass spectrometry determination of platinum metals in basic rocks and ores. J Anal Chem. 2019;74(1):33–40.
Tian L, Song X, Liu T, Li A, Ning Y, Hua X, Liang D. A review of spectroscopic probes constructed from aptamer–binding gold/silver nanoparticles or their dimers in environmental pollutants` detection. Anal Sci. 2022;38:1247–59.
Hagarová I, Nemček L, Šebesta M, Zvěřina O, Kasak P, Urík M. Preconcentration and separation of gold nanoparticles from environmental waters using extraction techniques followed by spectrometric quantification. Int J Mol Sci. 2022;23(19):11465.
Ermolin MS, Ivaneev AI, Brzhezinskiy AS, Karandashev VK, Mokhov AV, Fedotov PS. Anthropogenic source of gold in Moscow urban dust. J Anal Chem. 2022;77(10):1340–8.
Kubrakova IV, Nikulin AV, Koshcheeva IYa, Tyutyunnik OA. Platinum metals in the environment: content, determination, behaviour in natural systems. Chem Sustain Dev. 2012;20:593–603.
Volzhenin AV, Petrova NI, Medvedev NS, Irisov DS, Saprykin AI. Determination of gold and palladium in rocks and ores by atomic absorption spectrometry using two-stage probe atomization. J Anal Chem. 2017;72(2):156–62.
Filatova DG, Eskina VV, Baranovskaya VB, Vladimirova SA, Gaskov AM, Rumyantseva MN, Karpov YA. Determination of gold and cobalt dopants in advanced materials based on tin oxide by slurry sampling high-resolution continuum source graphite furnace atomic absorption spectrometry. Spectrochim Acta Part B At Spectrosc. 2018;140:1–4.
Petrov AM, Klimova OI, Dalnova OA, Karpov YA. Determination of gold and platinum metals in second-hand and technogenic materials with the use of the sorption atomic emission method with a multichannel emission spectra analyzer. Inorg Mater. 2014;50(14):1387–91.
Tao D, Guo W, Xie W, Jin L, Guo Q, Hu S. Rapid and accurate determination of gold in geological materials by an improved ICP-MS method. Microchem J. 2017;135:221–5.
Chao JB, Wang JR, Zhang JQ. Accurate determination and characterization of gold nanoparticles based on single particle—inductively coupled plasma-mass spectrometry. Chin J Anal Chem. 2020;48(7):946–54.
Ghosh M, Swain KK, Chavan TA, Wagh DN, Verma R. Determination of gold and silver in dross using EDXRF technique. Xray Spectrom. 2015;44(1):13–5.
Maksimova YA, Dubenskiy AS, Garmash AV, Pashkova GV, Shigapova IV, Seregina IF, Pavlova LA, Sharanov PYu, Bolshov MA. Simultaneous determination of Os, Ir, Pt and Au in sorbent phases by total reflection X-ray fluorescence. Spectrochim Acta Part B At Spectrosc. 2022;196:106521.
Mokhodoeva OB, Nikulin AV, Myasoedova GV, Kubrakova IV. A new combined ETAAS method for the determination of platinum, palladium, and gold traces in natural samples. J Anal Chem. 2012;67(6):531–6.
Bahadir Z. A surfactant-based emulsification microextraction (SBEME) method for the atomic absorption determination of gold. Desalin Water Treat. 2019;169:305–11.
Wang N, Sun X-D, Huo D. Determination of gold in mineral samples by flame atomic absorption spectrometry after the separation and preconcentration with small fire assay. Spectrosc Spectr Anal. 2019;39(8):2614–7.
Kannouma RE, Hammad MA, Kamal AH, Mansour FR. Miniaturization of liquid-liquid extraction; the barriers and the enablers. Microchem J. 2022;182: 107863.
El-Shahawi MS, Bashammakh AS, Bahaffi SO. Chemical speciation and recovery of gold(I, III) from wastewater and silver by liquid–liquid extraction with the ion-pair reagent amiloride mono hydrochloride and AAS determination. Talanta. 2007;72(4):1494–9.
Tang W, An Y, Row KH. Emerging applications of (micro) extraction phase from hydrophilic to hydrophobic deep eutectic solvents: opportunities and trends. TrAC Trends Anal Chem. 2021;136:116187.
Płotka-Wasylka J, de la Guardia M, Andruch V, Vilková M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem J. 2020;159:105539.
Omar KA, Sadeghi R. Physicochemical properties of deep eutectic solvents: a review. J Mol Liq. 2022;360:119524.
Yuan Z, Liu H, Yong WF, She Q, Esteban J. Status and advances of deep eutectic solvents for metal separation and recovery. Green Chem. 2022;24(5):1895–929.
Wazeer I, Hizaddin HF, Hashim MA, Hadj-Kali MK. An overview about the extraction of heavy metals and other critical pollutants from contaminated water via hydrophobic deep eutectic solvents. J Environ Chem Eng. 2022;10(6):108574.
Geng Y, Xiang Z, Lv C, Wang N, Wang Y, Yang Y. Recovery of gold from hydrochloric medium by deep eutectic solvents based on quaternary ammonium salts. Hydrometallurgy. 2019;188:264–71.
Yılmaz Ö, Durak BY, Tekin Z, Koçoğlu ES, Bakırdere S. Accurate and precise determination of gold in plating bath solution: deep eutectic solvent based liquid phase microextraction—slotted quartz tube—flame atomic absorption spectrometry. At Spectrosc. 2019;53(2):165–73.
Mokhodoeva O, Maksimova V, Shishov A, Shkinev V. Separation of platinum group metals using deep eutectic solvents based on quaternary ammonium salts. Sep Purif Technol. 2023;305:122427.
Fernández M, Boiteux J, Espino M, Gomez FJV, Silva MF. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal Chim Acta. 2018;1038:1–10.
Strzemski M, Dresler S, Podkościelna B, Skic K, Sowa I, Załuski D, Verpoorte R, Zielińska S, Krawczyk P, Wójciak M. Effectiveness of volatile natural deep eutectic solvents (VNADESs) for the green extraction of chelidonium majus isoquinoline alkaloids. Molecules. 2022;27(9):2815.
Rye TK, Martinovic G, Eie LV, Hansen FA, Halvorsen TG, Pedersen-Bjergaard S. Electromembrane extraction of peptides using deep eutectic solvents as liquid membrane. Anal Chim Acta. 2021;1175: 338717.
Liu R, Hao J, Wang Y, Meng Y, Yang Y. A separation strategy of Au(III), Pd(II) and Pt(IV) based on hydrophobic deep eutectic solvent from hydrochloric acid media. J Mol Liq. 2022;365: 120200.
Karandashev VK, Khvostikov VA, Nosenko SV, Burmii ZhP. Stable highly enriched isotopes in routine analysis of rocks, soils, grounds, and sediments by ICP-MS. Inorg Mater. 2017;53(14):1432–41.
Schaeffer N, Abranches DO, Silva LP, Martins MAR, Carvalho PJ, Russina O, Triolo A, Paccou L, Guinet Y, Hedoux A, Coutinho JAP. Non-ideality in thymol + menthol type V deep eutectic solvents. ACS Sustain Chem Eng. 2021;9(5):2203–11.
Shishov A, Shakirova F, Markova U, Tolstoy P, Bulatov A. A new hydrophobic deep eutectic solvent based on thymol and 4-(dimethylamino)benzaldehyde: derivatization and microextraction of urea. J Mol Liq. 2022;353:118820.
Krylov VA, Krylov AV, Mosyagin PV, Matkivskaya YuO. Liquid-phase microextraction preconcentration of impurities. J Anal Chem. 2011;66:331–50.
King SR, Massicot J, McDonagh AM. A straightforward route to tetrachloroauric acid from gold metal and molecular chlorine for nanoparticle synthesis. Metals. 2015;5(3):1454–61.
Tonello NV, D’Eramo F, Marioli JM, Crevillen AG, Escarpa A. Extraction-free colorimetric determination of thymol and carvacrol isomers in essential oils by pH-dependent formation of gold nanoparticles. Microchim Acta. 2018;185:352.
Busev AI, Ivanov VM. Analytical chemistry of gold. Moscow: Nauka; 1973. (in Russian).
Wang C, Yu C. Detection of chemical pollutants in water using gold nanoparticles as sensors: a review. Rev Anal Chem. 2012;32(1):1–14.
De La Calle I, Pena-Pereira F, Cabaleiro N, Lavilla I, Bendicho C. Ion pair-based dispersive liquid–liquid microextraction for gold determination at ppb level in solid samples after ultrasound-assisted extraction and in waters by electrothermal-atomic absorption spectrometry. Talanta. 2011;84(1):109–15.
Ashkenani H, Taher MA. Use of ionic liquid in simultaneous microextraction procedure for determination of gold and silver by ETAAS. Microchem J. 2012;103:185–90.
Kim M, Tudino MB. Evaluation of performance of three different hybrid mesoporous solids based on silica for preconcentration purposes in analytical chemistry: from the study of sorption features to the determination of elements of group IB. Talanta. 2010;82:923–30.
Tuzen M, Saygi KO, Soylak M. Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater. 2008;156:591–5.
Soylak M, Unsal YE. Double-walled carbon nanotubes as a solid phase extractor for separation preconcentration of traces of gold from geological and water samples. Int J Environ Anal Chem. 2011;91:440–7.
Liu R, Liang P. Determination of gold by nanometer titanium dioxide immobilized on silica gel packed microcolumn and flame atomic absorption spectrometry in geological and water samples. Anal Chim Acta. 2007;604:114–8.
Płotka-Wasylka J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta. 2018;181:204–9.
Acknowledgements
This work was supported by the Ministry of Science and High Education of the Russian Federation [GEOKHI RAS].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests relevant to the content of this article to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Maksimova, V., Lapina, V., Martynov, L. et al. Gold Determination by Electrothermal Atomic Absorption Spectrometry After Preconcentration Using Natural Deep Eutectic Solvent Based on Menthol and Camphor. J. Anal. Test. 7, 435–443 (2023). https://doi.org/10.1007/s41664-023-00279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-023-00279-7