Abstract
Monitoring the intracellular adenosine triphosphate (ATP) level is vital for elaborating its function in physiological states. However, the intracellular fluorescence sensing of ATP using ATP aptamer remains difficult owing to non-target displacement and susceptibility toward enzymatic degradation. Herein, by combining 2D Cu-MOF nanosheets and FAM labelled ATP aptamer, we developed a fluorescent Cu-MOFs/aptamer nanoprobe to image and sense intracellular ATP. This nanoprobe reveals a very low fluorescence intensity because of the excellent quenching efficiency of 2D Cu-MOF nanosheets. The presence of ATP was capable to dissociate the FAM-aptamer from Cu-MOF nanosheets and resulted in an intense fluorescence signal. The Cu-MOFs/aptamer nanoprobe enables highly sensitive and selective measurement of ATP level ranging from 10 μM to 800 μM with a detection limit of 4.24 μM. This nanosystem also further realized in situ detection of the undulation of ATP trigged by drug stimulation, depending on the selective delivery of the nanoprobe and attractive capability of resisting nonspecific displacement. The constructed nanoprobe may supply a potential platform in clinical diagnostics and biological studies.
Graphical Abstract

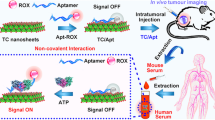
Two-dimension Cu-MOFs nanosheets-aptamer nanoprobe was developed for in situ monitoring the change of ATP in living cells.







Similar content being viewed by others
References
Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–69.
Higgins CF, Hiles ID, Salmond GP, Gill DR, Downie JA, Evans IJ, Holland IB, Gray L, Buckel SD, Bell AW, Hermodson MA. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323(6087):448–50.
Geng X, Sun Y, Guo Y, Zhao Y, Zhang K, Xiao L, Qu L, Li Z. Fluorescent carbon dots for in situ monitoring of lysosomal ATP levels. Anal Chem. 2020;92(11):7940–6.
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29.
Przedborski S, Vila M. MPTP: a review of its mechanisms of neurotoxicity. Clin Neurosci Res. 2001;1(6):407–18.
Li X, Guo X, Cao L, Xun Z, Wang S, Li S, Li Y, Yang G. Water-soluble triarylboron compound for ATP imaging in vivo using analyte-induced finite aggregation. Angew Chem Int Ed Engl. 2014;53(30):7809–13.
Zhou Z, Du Y, Dong S. Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label-free aptamer sensor. Anal Chem. 2011;83(13):5122–7.
Ng S, Lim HS, Ma Q, Gao Z. Optical aptasensors for adenosine triphosphate. Theranostics. 2016;6(10):1683–702.
Jiang K, Wu Y, Chen J, Shi M, Meng H-M, Li Z. Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples. Chinese Chem Lett. 2020. https://doi.org/10.1016/j.cclet.2020.11.031.
Meng HM, Zhang X, Lv Y, Zhao Z, Wang NN, Fu T, Fan H, Liang H, Qiu L, Zhu G, Tan W. DNA dendrimer: an efficient nanocarrier of functional nucleic acids for intracellular molecular sensing. ACS Nano. 2014;8(6):6171–81.
Jin F, Zheng J, Liu C, Yang S, Li Y, Li J, Lian Y, Yang R. Dual-stimuli responsive i-motif/nanoflares for sensing ATP in lysosomes. Analyst. 2014;139(15):3714–7.
Jia L, Ding L, Tian J, Bao L, Hu Y, Ju H, Yu JS. Aptamer loaded MoS2 nanoplates as nanoprobes for detection of intracellular ATP and controllable photodynamic therapy. Nanoscale. 2015;7(38):15953–61.
Wang Y, Li Z, Weber TJ, Hu D, Lin CT, Li J, Lin Y. In situ live cell sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal Chem. 2013;85(14):6775–82.
Wang Y, Li Z, Hu D, Lin CT, Li J, Lin Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J Am Chem Soc. 2010;132(27):9274–6.
Zhao M, Wang Y, Ma Q, Huang Y, Zhang X, Ping J, Zhang Z, Lu Q, Yu Y, Xu H, Zhao Y, Zhang H. Ultrathin 2D metal-organic framework nanosheets. Adv Mater. 2015;27(45):7372–8.
Maurin G, Serre C, Cooper A, Ferey G. The new age of MOFs and of their porous-related solids. Chem Soc Rev. 2017;46(11):3104–7.
Nath I, Chakraborty J, Verpoort F. Metal organic frameworks mimicking natural enzymes: a structural and functional analogy. Chem Soc Rev. 2016;45(15):4127–70.
Li H, Zheng B, Huang K-W. A new class of PN3-pincer ligands for metal-ligand cooperative catalysis. Coordin Chem Rev. 2015;293–294:116–38.
Fan Y, Zhang J, Shen Y, Zheng B, Zhang W, Huo F. Emerging porous nanosheets: from fundamental synthesis to promising applications. Nano Res. 2020;14(1):1–28.
Chen W-H, Yu X, Liao W-C, Sohn YS, Cecconello A, Kozell A, Nechushtai R, Willner I. ATP-responsive aptamer-based metal-organic framework nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv Funct Mater. 2017;27(37):1702102.
Li S, Tan L, Meng X. Nanoscale metal-organic frameworks: synthesis, biocompatibility, imaging applications, and thermal and dynamic therapy of tumors. Adv Funct Mater. 2020;30(13):1908924.
Rojas S, Arenas-Vivo A, Horcajada P. Metal-organic frameworks: a novel platform for combined advanced therapies. Coordin Chem Rev. 2019;188:202–26.
Meng HM, Hu XX, Kong GZ, Yang C, Fu T, Li ZH, Zhang XB. Aptamer-functionalized nanoscale metal-organic frameworks for targeted photodynamic therapy. Theranostics. 2018;8(16):4332–44.
Shi X, Meng HM, Geng X, Qu L, Li Z. DNAzyme-metal-organic framework two-photon nanoprobe for in situ monitoring of apoptosis-associated Zn2+ in living cells and tissues. ACS Sens. 2020;5(10):3150–7.
Meng HM, Shi X, Chen J, Gao Y, Qu L, Zhang K, Zhang XB, Li Z. DNA amplifier-functionalized metal-organic frameworks for multiplexed detection and imaging of intracellular mRNA. ACS Sens. 2020;5(1):103–9.
Qu F, Sun C, Lv X, You J. A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim Acta. 2018;185:359.
Li J, Zhang Y, Zou Z, Qing Z, Yang S, Yang J, Zhang L, Feng F, Yang R. MIL/aptamer as a nanosensor capable of resisting nonspecific displacement for ATP imaging in living cells. ACS Omega. 2019;4(5):9074–80.
Deng J, Wang K, Wang M, Yu P, Mao L. Mitochondria targeted nanoscale zeolitic imidazole framework-90 for ATP imaging in live cells. J Am Chem Soc. 2017;139(16):5877–82.
Zhou X, Li J, Tan LL, Li Q, Shang L. Novel perylene probe-encapsulated metal-organic framework nanocomposites for ratiometric fluorescence detection of ATP. J Mater Chem B. 2020;8(16):3661–6.
Wang K, Qian M, Qi H, Gao Q, Zhang C. Multifunctional zeolitic imidazolate framework-8 for real-time monitoring ATP fluctuation in mitochondria during photodynamic therapy. Nanoscale. 2020;12(29):15663–9.
Yi JT, Chen TT, Huo J, Chu X. Nanoscale zeolitic imidazolate framework-8 for ratiometric fluorescence imaging of MicroRNA in living cells. Anal Chem. 2017;89(22):12351–9.
Qiu Q, Chen H, Ying S, Sharif S, You Z, Wang Y, Ying Y. Simultaneous fluorometric determination of the DNAs of Salmonella enterica, Listeria monocytogenes and Vibrio parahemolyticus by using an ultrathin metal-organic framework (type Cu-TCPP). Microchim Acta. 2019;186:93.
Geng X, Li Z, Hu Y, Liu H, Sun Y, Meng H, Wang Y, Qu L, Lin Y. One-pot green synthesis of ultrabright n-doped fluorescent silicon nanoparticles for cellular imaging by using ethylenediaminetetraacetic acid disodium salt as an effective reductant. ACS Appl Mater Inter. 2018;10(33):27979–86.
Beis I, Newsholme EA. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975;152(1):23–32.
Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364.
Tan X, Chen T, Xiong X, Mao Y, Zhu G, Yasun E, Li C, Zhu Z, Tan W. Semiquantification of ATP in live cells using nonspecific desorption of DNA from graphene oxide as the internal reference. Anal Chem. 2012;84(20):8622–7.
Pu Y, Zhu Z, Han D, Liu H, Liu J, Liao J, Zhang K, Tan W. Insulin-binding aptamer-conjugated graphene oxide for insulin detection. Analyst. 2011;136(20):4138–40.
Liu Z, Chen S, Liu B, Wu J, Zhou Y, He L, Ding J, Liu J. Intracellular detection of ATP using an aptamer beacon covalently linked to graphene oxide resisting nonspecific probe displacement. Anal Chem. 2014;86(24):12229–35.
Xu Z, Singh NJ, Lim J, Pan J, Kim HN, Park S, Kim KS, Yoon J. Unique sandwich stacking of pyrene-adenine-pyrene for selective and ratiometric fluorescent sensing of ATP at physiological pH. J Am Chem Soc. 2009;131(42):15528–33.
Yi M, Yang S, Peng Z, Liu C, Li J, Zhong W, Yang R, Tan W. Two-photon graphene oxide/aptamer nanosensing conjugate for in vitro or in vivo molecular probing. Anal Chem. 2014;86(7):3548–54.
Ozkan A, Atar N, Yola ML. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: development of molecularly imprintedC SPR nanosensor for anticancer drug, etoposide. Biosens Bioelectron. 2019;130:293–8.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (21974125 and 21605038), the National 111 Project of China (D20003) and the Collaborative Innovation Project of Zhengzhou (Zhengzhou University) (Grant No. 18XTZX12002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
About this article
Cite this article
Shi, X., Xu, H., Wu, Y. et al. Two-Dimension (2D) Cu-MOFs/aptamer Nanoprobe for In Situ ATP Imaging in Living Cells. J. Anal. Test. 5, 165–173 (2021). https://doi.org/10.1007/s41664-021-00172-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-021-00172-1