Abstract
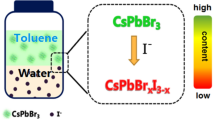
Cubic phase CsPbBr3 perovskite nanocrystals (PNCs) was prepared by a high-temperature hot-injection method. The high photoluminescence quantum yield (PLQY) of as-prepared CsPbBr3 PNCs was 87%, which can be used for the determination of chloridion in domestic water samples based on their wavelength-shift characteristics via halide exchange. The proposal approach for the determination of chloridion reveals a linear correlation ranged from 10 to 200 μM of the chloridion concentration and the wavelength shift of CsPbBr3 PNCs with a correlation coefficient of R2 = 0.9956. The as-mentioned method reveals neglectable responses towards those co-existing ions in the water aside from chloridion, due to the quick exchange between Cl and Br and the outstanding color change caused by wavelength shift. The strategy has been applied to the determination of chloridion in water samples with the recoveries of 98.9–104.2% and the limit of detection (LOD) of 4 μM. These results show that the suggested approach is promising for the development of novel fluorescence detection for chloridion in water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water resource, an indispensable element in the world, has great relation with the ecological environment and public health. Moreover, the available natural water for exploitation is extremely limited, and always full of bacteria and fungi residual. In hence, the utilization of natural water is restricted and effective treatment to decrease the existence of bacteria and fungi are necessary. Sterilization process in the treatment of natural water has enabled the usability of the target and decrease of the number of bacteria. Chloridion, a familiar anion, has played an essential role and showed better efficiency towards many kinds of bacteria in the sterilization process. The underexercise chloridion will cause potential impact on water quality, too little chloridion will bring about an incomplete inhibit of bacteria, while excessive chloridion cause damage to human skin, respiratory and nervous system [1,2,3]. Therefore, a highly attractive method to detect chloridion in domestic water must be taken into account. Rapidly developing chloridion detection techniques, such as iodometry, colorimetry, electrochemical sensors and ion chromatography, provide potential solutions for the control of chloridion in domestic water. However, these widespread methods mainly require complicated pretreatment and equipment, resulting in longer detection time or expensive cost [4,5,6,7,8]. Fluorescence sensing methods based on nanomaterials are of low cost, easy operation, fast response and high efficiency that serve in the detection of numerous ions and micro-molecule [9,10,11].
With the fluorescence sensing progresses, lead-based halide perovskite nanocrystals (PNCs, formed APbX3 (A = MA+, FA+, Cs+, et al.; X = Cl−, Br−, I−) have received significant attention owing to their outstanding properties such as high photoluminescence quantum yields (PLQYs), narrow full width at half-maximum (FWHM), tunable emission wavelengths and high defect-tolerant nature [12,13,14,15,16,17,18,19,20,21]. Furthermore, the excellent PLQYs and narrow FWHM of PNCs have endowed their attractive preponderance, and established higher efficiency for the fluorescence sensing technology in analytical chemistry. The fluorescence sensing approaches using PNCs have been successfully applied in the determination of heavy metal ions, micromolecule and gases [22,23,24,25,26,27,28]. CsPbBr3, a kind of PNCs with excellent optical properties and better halogen exchange, can promote the exchange between chloridion and CsPbBr3 PNCs and generate CsPbClxBr3–x PNCs. The process of halogen exchange is accompanied by obvious color change due to its luminescence wavelength blue shift simultaneously [29, 30], and therefore, an efficient fluorescence sensing approach for chloridion can be designed.
In this study, a fluorescence sensing approach for chloridion has been constructed based on the halogen exchange between chloridion and CsPbBr3 PNCs. In addition to the acceleration of halogen-exchange process through vortex-assisted oscillation, the other sensing conditions were also studied and optimized. The method has been applied in the determination of chloridion in domestic water with the characteristics of high efficient and straightforward, which can be completed within half a minute.
2 Experimental
2.1 Materials
Lead bromide (PbBr2, 99%), cesium carbonate (Cs2CO3, 99%), octadecene (ODE, > 90%), tetrabutylammonium bromide (TBA-Br, 99%), tetrabutylammonium chloride (TBA-Cl, 97%), sodium hypochlorite (NaClO, AR), sodium fluoride (NaF, 98%), potassium carbonate (K2CO3, 99%), sodium carbonate anhydrous (Na2CO3, 99%), magnesium sulfate anhydrous (Mg2SO4, > 99.5%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, AR), oleic acid (OA, 90%), oleylamine (OLAM, 80–90%), toluene (C7H8, > 99.5%), hexane (C6H14, > 99.5%) and ethyl acetate (C4H8O2, > 99.5%) were all purchased from Shanghai Aladdin Bio-Chem Technology Co. (China), and the other chemicals were used without further purification in this work.
2.2 Preparation of CsPbBr3 PNCs
The preparation of CsPbBr3 PNCs using high-temperature hot-injection was referenced to the report of Kovalenko [12]. In the preparation, 15 mL ODE, 300 mg Cs2CO3 and 1 mL OA were sequentially loaded into a three-necked flask under N2 atmosphere and the temperature was raised to 150 °C to yield the faint yellow Cs-OA. In another three-necked flask, PbBr2 precursor was prepared by mixed 10 mL ODE, 0.4 mM PbBr2, 1 mL OA and 1 mL OLAM. After completing solubilisation of PbBr2, the temperature was raised to 160 °C and 1 mL preheated Cs-OA solution was quickly injected. Finally, the high-quality CsPbBr3 PNCs can be obtained after purification with hexane/ethyl acetate for several times. The as-prepared CsPbBr3 PNCs was diluted with hexane to prepare a standard stock solution and stored for further use.
2.3 Determination of Chloridion
The complex solution containing 2 mL CsPbBr3 PNCs and 20 μL TBA-Cl, with H2SO4 used to adjust the pH to 1, was mixed into a centrifuge tube and drastic mechanic vibration at 1000 rpm for 30 s. After that, the color change was immediately observed by naked eyes with the concentrations of chloridion from 10 to 200 μM. The photoluminescence (PL) of CsPbBr3 PNCs accomplishing the anion exchange could be obtained by a fluorescence spectrophotometer. Subsequently, the method was established by investigating the relationship between the chloridion concentration and the wavelength shift of CsPbBr3 PNCs, and all experiments were retested three times.
2.4 Selectivity and Practical Sample Testing
The interference of co-existing ions in domestic water was studied following the similar procedure as the above. In the study, 50 μL aqueous solution containing 500 μM ion (Br−, F−, ClO−, K+, Na+, Mg2+ or Fe3+) was tested with the CsPbBr3 PNCs. Water samples were collected from the university campus, filtered with 0.22 μm filter to remove large particles, and stored for use. 50 μL prepared water samples were used to observe the wave-shifted fluorescence.
2.5 Characterization
Powder X-ray diffraction (XRD) patterns were recorded by a Rigaku Ultimate IV (Japan) diffractometer using Cu Kα radiation. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were collected on a Tecnai-G2-F30 transmission electron microscopes (FEI, USA) under 300 kV. X-ray photoelectron spectroscopy (XPS) was conducted on a Thermo Scientific™ K-Alpha™+ spectrometer equipped with a monochromatic Al Kα X-ray source (1486.6 eV) operating at 100 W. UV–Vis absorption spectroscopy was conducted on a UV-2600i UV–Vis spectrophotometer (Shimadzu, Japan). Fluorescence spectra were performed with an F-4500 fluorescence spectrophotometer (Hitachi, Japan), and PL decay curve was collected by the QM-TM spectrofluorometer (PTI, USA).
3 Results and Discussion
The characterization of CsPbBr3 PNCs was performed and shown in Fig. 1. The three-dimensional structure of cubic phase CsPbBr3 PNCs was composed of octahedral structure of [PbBr6]4−, bromine (I) anions and cesium (I) cation exhibited in Fig. 1a. The octahedral structure of [PbBr6]4− was composed of lead (II) cation and bromine (I) anions and formed via corner sharing. Cesium (I) cation was filled in the cubic center's vacancy to support the entire three-dimensional frame. The crystal phase of CsPbBr3 PNCs was confirmed by X-ray diffraction and shown in Fig. 1b. The diffraction peaks at 14.8°, 21.2°, 26.3°, 30.4°, 34.2°, 37.5° and 43.7° were corresponding to (100), (110), (111), (200), (210), (211) and (202) of cubic phase CsPbBr3 PNCs (PDF#18-0364), respectively. A typical transmission electron microscopy (TEM) image, as presented in Fig. 1c, indicating a cubic morphology CsPbBr3 PNCs with a mean diameter about 11.09 ± 1.98 nm (Fig. 1d). The high-resolution transmission electron microscopy (HRTEM) revealed the as-prepared CsPbBr3 PNCs with high crystallinity, and the interplanar spacing was 5.8 Å corresponding to (100) crystal of CsPbBr3 PNCs. The structure of CsPbBr3 PNCs changed from uniform to disorder gradually after halide exchanges with chloride in water. With the influence of H2O molecules, as shown in the TEM image of Fig. S1, the microstructures of CsPbClxBr3–x PNCs were agglomerated, destroyed and the size of some particles became larger.
Surface chemical composition and structure analysis of CsPbBr3 PNCs were carried out using X-ray photoelectron spectroscopy (Fig. 2a). The strong peaks in the XPS survey spectra included Cs3d (~ 725 and 738 eV), O1s (~ 532 eV), Pb4d (~ 413 and 437 eV), C1s (~ 285 eV), Pb4f (~ 138 and 143 eV), Br3d (~ 68 eV) and Pb5d (~ 20 eV). The presence of elemental C and O were probably related to the surface ligand OA. The fluorescence emission and the UV–Vis absorption spectra of CsPbBr3 PNCs were shown in Fig. 2b. A sharp emission peak at 513 nm, with a narrow full width at half-maximum (FWHM) of 28 nm, was found in the fluorescence emission spectra. Furthermore, a strong absorption peak at 488 nm could be found in the UV–Vis absorption spectra, which belonged to the band-edge absorption of CsPbBr3 PNCs. The CsPbBr3 PNCs emitted very bright green fluorescence (as shown in the inset of Fig. 2b, PLQYs ~ 87%) under the excitation at 365 nm. The emission dynamics decay curve of CsPbBr3 PNCs had been presented in Fig. 2c, and the average PL decay lifetime was 23.7 ns (triple-exponential decay function, τ = (A1τ12 + A2τ22 + A3τ32)/(A1τ1 + A2τ2 + A3τ3)). These results indicate that the radiation transition dominates the exciton recombination process, which makes CsPbBr3 PNCs exhibit excellent optical properties.
Several parameters, in the process of halogen exchange, including the dosage of the aqueous solution, pH, the stirring rate and time were investigated. According to previous reports [29], the phenomenon of ion exchange between Cl− with CsPbBr3 PNCs was not evident and without external force when it migrated from aqueous phase to organic. On the contrary, the vortex-assisted shock method increased the number of Cl− entering the interface of CsPbBr3 PNCs due to the aqueous-organic interface uniformly re-dispersed, which remarkably accelerated the ion exchange process between Cl− and Br−. The dosage of the aqueous solution and the value of pH were key factors on the process of halogen exchange. Considering the adverse impact of aqueous solution on the structure of CsPbBr3 PNCs, Vn-hexane/\(V_{\text{H}_{2}\text{O}}\) = 100/1 was chosen for the further study. Furthermore, the chloridion exchange occurred easily in a short time under a condition of strong acidic, and pH 1 was selected as the optimal in our work (Fig. S2). It can be observed that the ion exchange process could be completed within 30 s as displayed in Fig. 3a and b, when the stirring rate of vortex-assisted shock changed from 750 to 1250 rpm (Ccl− = 200 μM). However, it could be observed that the ion exchange rate did not increase significantly as the stirring rate over 1000 rpm. In hence, 1000 rpm of stirring rate and 30 s of reaction time were selected as the optimal conditions in our work. After that, the selectivity of the fluorescence sensing approach was explored to confirm the practical application potential. The wavelength shifts of Br−, F−, ClO−, K+, Na+, Mg2+ and Fe3+ in the same concentration (500 μM) were displayed in Fig. 3c. Compared with Cl−, the response of this sensing method to these ions is negligible, indicating that it has high specificity towards Cl−.
Under the optimal conditions, the wavelength shift and correlation relationship of CsPbBr3 PNCs induced by different concentrations of Cl− were studied. As shown in Fig. 4a, the fluorescence spectra of the CsPbBr3 PNCs presented a continuous blue shift as the Cl− concentration increased, and the central peak wavelength moved from 513 to 483 nm (Δλ = 30 nm). Meanwhile, the band-edge absorption of CsPbBr3 PNCs shifted from 488 to 463 nm (Fig. 4b). Under the visible light, as shown in Fig. 4c, the distinct color of CsPbBr3 PNCs changed significantly, from cyan to reseda as the Cl− concentration increased. When exposed at 365 nm UV light, the fluorescence color of CsPbBr3 PNCs changed from green to blue–green, and finally to blue. The correlation relationship between Cl− concentration in the range of 10–200 μM and the wavelength shift of CsPbBr3 PNCs was established, and the wavelength shift was enhanced from 4 to 30 nm with a regression equation of y = 0.1414x + 1.895, where y was the wavelength shift of CsPbBr3 PNCs and x was the concentration of Cl−. The correlation coefficient (R2) was 0.9956 and the limit of detection was calculated to be 4.0 μM (LOD = 3 s/k; s = 0.1897, the standard deviation given by 10 parallel blank measurements; k, the slope of the correlation function relationship). The LOD was lower than the national security standard (0.3–0.5 mg/L) (GB5749-2006), verifying that our approach can be used for the qualitative and quantitative determination of Cl−.
Effects of different concentrations of Cl− on the wavelength shift of CsPbBr3 PNCs in a fluorescence emission and b UV–Vis absorption spectra; c the apparent color change of CsPbBr3 PNCs under visible light (up) and UV light (down) with adding different concentrations of Cl−; d linear relationship between the wavelength shifts and the concentrations of Cl−
The concentration of Cl− in different water samples were determined, and the results are listed in Table 1 based on the linear relationship established in above experiment. The recoveries were between 98.9 and 104.2%, and the relative standard deviations (RSDs) were less than 8.8%. Furthermore, the fluorescence wavelength-shift colorimetric approach via halide exchange of CsPbBr3 PNCs showed a wider linear range and lower LOD than those of reported methods (Table S1). The above experimental data proved that halogen ion exchange based on CsPbBr3 PNCs could be a practical and straightforward approach to detect the Cl− in domestic water samples.
4 Conclusion
In the present work, the CsPbBr3 PNCs with excellent optical properties were prepared via a high-temperature hot-injection approach. The as-prepared CsPbBr3 exhibited cubic phase structure, high photoluminescence quantum yields and a quick halogen exchange between chloridion and CsPbBr3 PNCs. The established method had shown a wavelength shift of CsPbBr3 PNC with the concentration change of chloridion. This method has high selectivity and sensitivity, and exhibits good linearity, low LODs, remarkable repeatability and excellent recovery in the determination of chloridion in domestic water samples within 30 s under a vortex-assisted reaction.
References
Cook C, Bakker K. Water security: debating an emerging paradigm. Global Envir Change. 2012;22:94–102.
Dong Y, Li G, Zhou N, Wang R, Chi Y, Chen G. Graphene quantum dot as a green and facile sensor for free chlorine in drinking water. Anal Chem. 2012;84:8378–82.
Nimkerdphol K, Nakagawa M. Effect of sodium hypochlorite on zebrafish swimming behavior estimated by fractal dimension analysis. J Biosci Bioeng. 2008;105:486–92.
Rahbar M, Paull B, Macka M. Instrument-free argentometric determination of chloride via trapezoidal distance-based microfluidic paper devices. Anal Chim Acta. 2019;1063:1–8.
Hallaj T, Amjadi M, Manzoori JL, Shokri R. Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim Acta. 2015;182:789–96.
Chango G, Palacio E, Cerdà V. Potentiometric chip-based multipumping flow system for the simultaneous determination of fluoride, chloride, pH, and redox potential in water samples. Talanta. 2018;186:554–60.
Moberg L, Karlberg B. An improved N, N’-diethyl-p-phenylenediamine (DPD) method for the determination of free chlorine based on multiple wavelength detection. Anal Chim Acta. 2000;407:127–33.
Robaina NF, Feiteira FN, Cassella AR, Cassella RJ. Determination of chloride in brazilian crude oils by ion chromatography after extraction induced by emulsion breaking. J Chromatogr A. 2016;1458:112–7.
Zhou M, Guo J, Yang C. Ratiometric fluorescence sensor for Fe3+ ions detection based on quantum dot-doped hydrogel optical fiber. Sens Actuators B. 2018;264:52–8.
Wang S, Huang Y, Zhang L, Li F, Lin F, Wang Y, Chen X. Highly selective fluorescence turn-on determination of Pb(II) in Water by in-situ enrichment of Pb(II) and MAPbBr3 perovskite growth in sulfydryl functionalized mesoporous alumina film. Sens Actuators B. 2021;326:128975.
Liu Y, Tang X, Zhu T, Deng M, Ikechukwu IP, Huang W, Yin G, Bai Y, Qu D, Huang X, Qiu F. All-inorganic CsPbBr3 perovskite quantum dots as photoluminescent probe for ultrasensitive Cu2+ detection. J Mater Chem C. 2018;6:4793–9.
Protesescu L, Yakunin S, Bodnarchuk MI, Krieg F, Caputo R, Hendon CH, Yang RX, Walsh A, Kovalenko MV. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15:3692–6.
Li X, Wu Y, Zhang S, Cai B, Gu Y, Song J, Zeng H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv Funct Mater. 2016;26:2435–45.
Zhang F, Zhong H, Chen C, Wu X, Hu X, Huang H, Han J, Zou B, Dong Y. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X=Br, I, Cl) quantum dots: potential alternatives for display technology. ACS Nano. 2015;9:4533–42.
Imran M, Caligiuri V, Wang M, Goldoni L, Prato M, Krahne R, Trizio LD, Manna L. Benzoyl halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J Am Chem Soc. 2018;140:2656–64.
Wang Y, Li X, Nalla V, Zeng H, Sun H. Solution-processed low threshold vertical cavity surface emitting lasers from all-inorganic perovskite nanocrystals. Adv Funct Mater. 2017;27:1605088.
Zhu X, Lin Y, Sun Y, Beard MC, Yan Y. Lead-halide perovskites for photocatalytic α-Alkylation of Aldehydes. J Am Chem Soc. 2019;141:733–8.
Qin C, Sandanayaka ASD, Zhao C, Matsushima T, Zhang D, Fujihara T, Adachi C. Stable room-temperature continuous-wave lasing in quasi-2D perovskite films. Nature. 2020;585:53–7.
Chen Q, Wu J, Ou X, Huang B, Almutlaq J, Zhumekenov AA, Guan X, Han S, Liang L, Yi Z, Li J, Xie X, Wang Y, Li Y, Fan D, Teh DBL, All AH, Mohammed OF, Bakr OM, Wu T, Bettinelli M, Yang H, Huang W, Liu X. All-inorganic perovskite nanocrystal scintillators. Nature. 2018;561:88–93.
Huang G, Li F, Cai Z, Zhang M. Synthesis of highly luminescent CsPbxMn1–xCl3 perovskite nanocrystals via using metal-organic Mn-Complex as precursor. J Alloys Compd. 2019;791:621–7.
Jung EH, Jeon NJ, Park EY, Moon CS, Shin TJ, Yang TY, Noh JH, Seo J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature. 2019;567:511–5.
Lu LQ, Tan T, Tian XK, Li Y, Deng P. Visual and sensitive fluorescent sensing for ultratrace mercury ions by perovskite quantum dots. Anal Chim Acta. 2017;986:109–14.
Huang S, Guo M, Tan J, Geng Y, Wu J, Tang Y, Su C, Lin CC, Liang Y. Novel fluorescence sensor based on all-inorganic perovskite quantum dots coated with molecularly imprinted polymers for highly selective and sensitive detection of omethoate. ACS Appl Mater Interfaces. 2018;10:39056–63.
Pramanik A, Gates K, Patibla S, Davis D, Begum S, Iftekhar R, Alamgir S, Paige S, Porter MM, Ray PC. Water-soluble and bright luminescent cesium-lead- bromide perovskite quantum dot-polymer composites for tumor-derived exosome imaging. ACS Appl Bio Mater. 2019;2:5872–9.
Chen X, Hu H, Xia Z, Gao W, Gou W, Qu Y, Ma Y. CsPbBr3 perovskite nanocrystals as highly selective and sensitive spectrochemical probes for gaseous HCl detection. J Mater Chem C. 2017;5:309–13.
Chen C, Cai Q, Luo F, Dong N, Guo L, Qiu B, Lin Z. Sensitive fluorescent sensor for hydrogen sulfide in rat brain microdialysis via CsPbBr3 quantum dots. Anal Chem. 2019;91:15915–21.
Tan L, Guo M, Tan J, Geng Y, Huang S, Tang Y, Su C, Lin C, Liang Y. Development of high-luminescence perovskite quantum dots coated with molecularly imprinted polymers for pesticide detection by slowly hydrolysing the organosilicon monomers in situ. Sens Actuators B. 2019;291:226–34.
Huang Y, Wang S, Zhu Y, Li F, Jin J, Dong J, Lin F, Wang Y, Chen X. Dual-mode of fluorescence turn-on and wavelength-shift for methylamine gas sensing based on space-confined growth of methylammonium lead tribromide perovskite nanocrystals. Anal Chem. 2020;92:5661–5.
Zhu Y, Li F, Huang Y, Lin F, Chen X. Wavelength-shift-based colorimetric sensing for peroxide number of edible oil using CsPbBr3 perovskite nanocrystals. Anal Chem. 2019;91:14183–7.
Akkerman QA, Innocenzo VD, Accornero S, Scarpellini A, Petrozza A, Prato M, Manna L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J Am Chem Soc. 2015;137:10276–81.
Acknowledgements
This research work is financially supported by the National Natural Science Foundation of China (22004105), special project of the Marine and Fishery Department of Xiamen (No. 19CZB001HJ03), and the Training Program of the Outstanding Young Scientific Talents in Fujian (2018-47).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Huang, GB., Guo, ZY., Ye, TX. et al. Colorimetric Determination of Chloridion in Domestic Water Based on the Wavelength Shift of CsPbBr3 Perovskite Nanocrystals via Halide Exchange. J. Anal. Test. 5, 3–10 (2021). https://doi.org/10.1007/s41664-021-00160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-021-00160-5