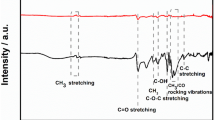
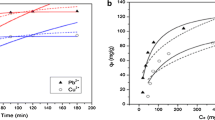
Abstract
Adsorptive stripping voltammetry using a glassy carbon electrode modified by a film of aminosepiolite was utilized for simultaneous pre-concentration and trace detection of cadmium, lead, and mercury ions in aqueous solution. The modified sepiolite exploited as electrode material was obtained by grafting on its surface of [(3-(2-aminoethylamino)propyl)]trimethoxysilane (AEPTMS). The results demonstrated that the amine groups on sepiolite efficiently affected the voltammetric detection of heavy metals. The full factorial design matrix and response surface methodology were applied in designing experiments, to determine the optimal conditions and to evaluate their mutual interactions. The high values of adjusted R2 obtained of the fitted model show that the experiments data were well explained by the model, which then allowed to acquire optimum parameters for the electroanalysis and detection of the analytes by differential pulse voltammetry. At pH 6.5 of accumulating medium, with an electrolysis potential of –0.9 V and in the concentration range of 10−8 M to 10−9 M, calibration plots were obtained. The limits of detection (3Sd/m) were 8.689 × 10−10 M, 8.197 × 10−10 M, and 8.099 × 10−10 M, respectively, for Cd2+, Pb2+, and Hg2+ ions. The interference effect of several cations and anions on the response of the analytes was also evaluated, and finally, the sensor was applied to the simultaneous detection of metal ions in tap water with satisfactory recovery rates.





Similar content being viewed by others
References
Tonle IK, Ngameni E, Tchieno MMF, Walcarius A. J Solid State Electrochem. 2015;19:1949.
Zhang S, He P, Lei W, Zhang G. J Electroanal Chem. 2014;724:29.
Azad UP, Turllapati S, Rastogi PK, Ganesan V. Electrochim Acta. 2014;127:193.
Colilla M, Darder M, Aranda P, Ruiz-Hitzky E. Chem Mater. 2005;17:708.
Ozkan D, Kerman K, Meric B, Kara P, Demirkan H, Polverejan M, Pinnavaia TJ, Ozsoz M. Chem Mater. 2002;14:1755.
Filho NLD. do Carmo DR. Talanta. 2006;68:919.
Dong YP, Ding Y, Zhou Y, Chen J, Wang CM. J Electroanal Chem. 2014;717:206.
Filho NLD, Okajima GL, Pires G, Costa RM, do Carmo R, Rosa AH. Port Electrochim Acta. 2008;26:163.
Nguelo BB, Kenne DG, Tonle IK, Ngameni E, Detellier C. Electroanalysis. 2018;30:543.
Tonle IK, Letaief S, Ngameni E, Walcarius A, Detellier C. Electroanalysis. 2011;23:245.
Jieumboue AT, Ngameni E, Tonle IK, Walcarius A. Chem Mater. 2009;21:4111.
Tonle IK, Ngameni E, Walcarius A. Sens Actuators B Chem. 2005;110:195.
Background document for development of WHO. Guidelines for drinking-water quality (2011). WHO/SDE/WSH/03.04/09/Rev/1.
Background document for development of WHO Guidelines for Drinking-Water Quality (2005). -9WHO/SDE/WSH/05.08/10.
Durkalec M, Szkoda J, Kolacz R, Opalinski S, Nawrocka A, Zmudzki J. Int J Environ Res. 2015;9:205.
Evans EH, Day JA, Palmer CD, Price WJ, Smith CMM, Tyson JF. J Anal Atmos Spectrom. 2005;2:562.
Da-Col JA, Domene SMA, Pereira-Filho ER. Food Anal Methods. 2009;2:110.
Sen I, Shandil A, Shrivastava VS. Adv Appl Sci Res. 2011;2:161.
Lachas H, Richaud R, Herod AA, Dugwell DR, Kandiyoti R, Jarvis KE. Analyst. 1999;124:177.
Okcu F, Ertas FN, Gokcul HI, Tural H. Turk J Chem. 2005;29:355.
Siriangkhawut W, Pencharee S, Grudpan K, Jakmunee J. Talanta. 2009;79:1118.
Wang J. Fresenius’ J Anal Chem. 1990;337:508.
Weuster-Botz D. J Biosci Bioeng. 2000;90:473.
Ymele E, Jiokeng ZLS, Tchieno MMF, Tonle IK. Adv Mater Sci. 2017;2:1.
Ensafi AA, Khayamian T, Atabati M. Talanta. 2003;59:727.
Gomez CG, Drogui P, Zaviska F, Seyhi B, Gortares-Moroyoqui P, Buelna G, Niera-Saenz C, Estrada-Alvardo M, Ulloa-Mercado RG. J Electroanal Chem. 2014;732:1.
Nde BD, Siriyabe M, Ahmed MM, Fon-Abi C, Paul Z, George NE, Kapseu C. J Chem Biol Phys Sci. 2014;3261.
Nde BD, Fon-Abi C, Tenin D, Kapseu C, Tchiegang C. Food Bioprocess Technol. 2012;5:108.
Yilmaz S, Ozturk B, Ozdemir D, Eroglu AE, Ertas FN. Turk J Chem. 2013;37:316.
Mannan S, Fakhru’l-Razi A, Alam MZ. J Environ Sci. 2007;19:23.
Heidari A, Younesi H, Mehraban Z. Chem Eng J. 2009;153:70.
Benhamou A, Baudu M, Derriche Z, Basly JP. J Hazard Mater. 2009;171:1001.
Ansanay-Alex S, Lomenech C, Hurel C, Marmier N. Int J Nanotechnol. 2012;9:204.
Acknowledgements
This work was supported by The World Academy of Sciences (TWAS) for the advancement of science in developing countries (Research Grant No. 16-515 RG/CHE/AF/AC_G-FR3240293302 allowed to I. K. Tonle).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ymélé, E., Jiokeng, S.L.Z., Nde, D.B. et al. Simultaneous Voltammetric Determination of Cd2+, Pb2+, and Hg2+ Ions Using Aminosepiolite-Coated Glassy Carbon Electrode: Optimization of Detection Parameters via Response Surface Methodology. J. Anal. Test. 3, 295–305 (2019). https://doi.org/10.1007/s41664-019-00086-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-019-00086-z