Abstract
Purpose
One strategy for treating cancer is to prevent metastatic spread. Matrix metalloproteinase are considered potential targets for cancer therapy because of their role in degrading the extracellular matrix and fostering tumor progression. In some cancer models, the small molecule 1,2,3,4,6-Penta-O-galloyl-β-d-glucose (PGG) exhibited inhibitory properties against matrix metalloproteinase (MMP) related metastatic activity. This study explored whether PPG may limit the potential for metastatic spread in oral squamous cell carcinoma.
Materials and methods
This study used Cal-27 cells, a cell line derived from a squamous cell carcinoma line of human tongue origin, and antibodies for MMP-1, -2, -3, -9, -13, MT1-MMP signal transducers and activators of transcription 3 (Stat3), and pStat3. Cells were treated with PGG at different concentrations to evaluate MMP and Stat3 activation. Expansion assays were performed using Matrigel matrixes to measure Cal-27 invasiveness in the presence of PGG.
Results
PGG decreased the expression of MMP-2, -9 and -13 in the Cal-27 cell line, decreased phosphorylation of Stat3 and reduced gene expression of MMP-2, -9 and -13. As observed in Matrigel expansion assays, PGG limited the invasiveness of Cal-27 cells in a dose-dependent manner.
Conclusion
PPG is a small molecule inhibitor with the potential to reduce the expression of the matrix metalloproteinases and to limit the invasiveness of the squamous cell carcinoma line, Cal-27. By controlling the expression of molecules responsible for metastasis, PPG may offer a new therapeutic option for treating oral squamous cell carcinoma.
Similar content being viewed by others
Introduction
Oral cancer is the 6th most common cancer worldwide with over 49,000 new cases expected in 2017 in the United States alone. Cancer of the oral cavity and oropharynx results in approximately 9700 deaths each year, with only a 50–60% 5-year survival rate [1]. One reason for the poor outcomes associated with oral cancer is that a large percentage of cases are first diagnosed in an advanced stage [2]. Tumor biology is an extremely complex process and oral carcinogenesis involves a series of concerted steps including precancerous lesions, invasion, and metastasis [3]. In addition to early diagnosis, research to develop new therapies targeted at tumor spread represent a potential strategy to lessen morbidity and mortality for those affected by oral squamous cell carcinoma (OSCC).
Matrix metalloproteinases and the tumor microenvironment
Matrix metalloproteinases (MMPs) are calcium-dependent, zinc-containing endopeptidases with a vast proteolytic potential that includes: collagen types I–XVII, pro forms of inflammatory molecules such as tumor necrosis factor (TNF), interleukin 1β (IL-1β), monocyte chemoattractant protein (MCP) and other extracellular matrix (ECM) components [4]. When present in excess, MMPs severely compromise tissue function and integrity and can worsen inflammatory conditions such as periodontal disease and arthritis [5, 6].
In recent years, the concept of the tumor microenvironment (TME) has emerged as an integral aspect of carcinogenesis [6]. The TME contains several cell types such as macrophages, T cells, and carcinoma-associated fibroblasts (CAF) that often coevolve with a tumor. These cells appear to provide many of the signals that trigger the pleiotropic properties of cancer cells. As the disease progresses, CAF, cancer cells and macrophages also secrete factors such as MMPs that contribute to tumor invasiveness [6].
MMPs are proteolytic enzymes that play a critical role in extracellular matrix (ECM) degradation and alter cell–cell adhesion within the TME [7,8,9] The basement membrane serves as a natural first barrier to tumor invasion and many of its components, such as collagen IV, laminin, and fibronectin become dysregulated in oral squamous cell carcinoma (OSCC) [10, 11]. Degradation of the ECM combined with altered cellular adhesion and mobility facilitates tumor invasion and metastatic spread.
MMPs also appear to be involved in angiogenesis and regulation of pro-growth and anti-growth signals. Several solid tumors exhibit overexpression of MMPs [7, 10, 11]. MMP-2 and MMP-9 degrade collagen IV while MMP-13 is highly efficient at cleaving fibronectin [12]. Research shows that OSCC tumor cells produce MMP endogenously, and are also capable of utilizing MMPs produced by stromal cells [13]. Studies indicate that controlling MMP-2 and MMP-9 decrease both the invasiveness and migration in Cal 27 and Ca 9-22 cells and may even be used as prognosis markers [14,15,16,17,18].
Several studies also associate MMPs with tumor invasion and angiogenesis [19,20,21]. MMP-9 knockdown attenuates migration and invasiveness in gliomas and triple negative breast cancer cells [22]. MMP-2 and MMP-9 increase osteoclast recruitment in prostate cancer, facilitating colonization by metastatic cells [19]. In gastric cancer increased expression of MMP-2, -7 and -9 correlate with a higher tumor stage, higher invasion depth and presence of distant metastasis [23]. In ovarian and gastric cancer, neutrophil MMP-9 expression is directly correlated to the vascular endothelial growth factor (VEGF), which governs the angiogenesis needed to sustain tumor growth [24, 25].
Poly-galloyl-glucopyranose
Poly-galloyl-glucopyranose or 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG), is a polyphenolic gallotannin synthesized by plants. Initially extracted from Rhus typhina (sumac) in 1990 by Hofmann and Gross, studies indicate that PGG has antidiabetic, antioxidant, anti-cancer and anti-inflammatory activities [26].
PGG was initially considered as an inflammatory promoter, since treating peripheral blood mononuclear cells (PBMCs) with PGG enhanced production of TNF-α and IL-1β. These studies suggested that inducing endogenous cytokines served as the mechanism by which PGG controls the proliferation of cancer cells, specifically S-180 sarcoma [27, 28]. This effect was more prominent for IL-1β, although it appears that levels of TNF-α that correlate more closely with anti-cancer activity.
Several hepatic cancer cell lines showed antiproliferative effects after treatment with low doses (10 µM) of PPG through the induction of glycine N-methyltransferase, an enzyme that protects against carcinogens such as aflatoxin and polyaromatic hydrocarbons. In Huh7, PGG at higher doses (20–100 µM) decreased colony formation and exhibited a proapoptotic effect in a dose-dependent manner [29]. More importantly, PGG decreases the epidermal growth factor-induced (EGF-induced) MMP-9 expression in prostate cancer cells, apparently through abrogation of JNK [30].
Since PGG affects the production and activity of MMPs, it might have a modulating effect on cancer cell invasion. Using the squamous carcinoma cell line Cal 27, this study sought to demonstrate that the small molecule PGG can inhibit the expression of clinically relevant MMPs by Cal 27 cells, potentially impairing cancer cell spread and the progression of oropharyngeal cancer.
Materials and methods
Cell culture
Squamous cell carcinoma cells (Cal 27) were purchased from ATCC (CRL-2095, VA, USA) and grown in DMEM with Glutamax, 10% FBS and Penicillin/Streptomycin (1055-024, 15140-122, 10438-018 Gibco-ThermoFischer Scientific, MA, USA) and kept at 37 °C in a humidified air chamber with 5% CO2. Cells were seeded at 3 × 105 cells/flask for the experiments and then grown to confluency. Cells between 3 and 10 passages were used for all experiments.
1,2,3,4,6-Penta-O-galloyl-β-d-glucose (G7548, Sigma-Aldrich, MO, USA) was dissolved in dimethylsulfoxide (DMSO) (D2650, Sigma-Aldrich, MO, USA) to obtain a 100 mM stock solution. Subsequent dilutions were done using water, and no flask contained more than 0.5 µL of DMSO (0.01%). Cells were simultaneously induced with PGG at concentrations of 1 µM, 2.5 µM, 5 µM or 10 µM according to results Figs. 1, 2, 3, 4, and then incubated for 48 h. The induction pattern followed similar methods for this cancer cell line, as described in previous studies. We selected the PGG concentrations based on published literature describing PGG effects in several cell lines [26,27,28, 31].
PGG prevents the upregulation of MMP-2, MMP-9 and MMP-13Cal-27 cells (squamous cell carcinoma) were treated with PGG at different concentrations for 48 h as described in the figure. a MMP-2 expression was not significantly different to the non-treated group (NT) when cells were treated with 1, 2.5 or 5 μM PGG (p > 0.05, n = 4) (one-way ANOVA, Bonferroni). MMP-2 expression was significantly decreased when Cal-27 cells were treated with 10 μM PGG (p < 0.01, n = 4) (one-way ANOVA, Bonferroni). Samples were obtained from cell media. b MMP-9 expression was not significantly different to the non-treated group (NT) when cells were treated with 1 or 2.5 μM PGG (p > 0.05, n = 4) (one-way ANOVA, Bonferroni). MMP-9 expression was significantly decreased when Cal-27 cells were treated with 5 or 10 μM PGG (p < 0.05 and p < 0.01 respectively, n = 4) (one-way ANOVA, Bonferroni). Samples were obtained from cytoplasmic protein extraction and normalized to Histone H3. c MMP-13 expression was not significantly different to the non-treated group (NT) when cells were treated with 1, 2.5 or 5 μM PGG (p > 0.05, n = 4) (one-way ANOVA, Bonferroni). MMP-13 expression was significantly decreased when Cal-27 cells were treated with 10 μM PGG (p < 0.01, n = 4) (one-way ANOVA, Bonferroni). Samples were obtained from cytoplasmic protein extraction and normalized to Histone H3
1,2,3,4,6-Penta-O-galloyl-ß-d-glucose (PGG) decreases Stat3 phosphorylation production in Cal-27 cells (squamous cell carcinoma) were treated with PGG at different concentrations for 48 h as described in the figure. PGG treatment significantly decreases Stat3 phosphorylation (pStat3) (p < 0.0001, n = 4) (two-way ANOVA, Bonferroni). Non-phosphorylated Stat3 was significantly higher than Stat3 in non-treated Cal-27 cells (p < 0.0001, n = 4) (two-way ANOVA, Bonferroni). Samples were obtained from cytoplasmic protein extraction and normalized to Histone H3
mRNA expression of MMPs after treatment of Cal-27 cells with 1,2,3,4,6-penta-O-galloyl-ß-d-glucose (PGG) Graph shows expression of MMP-2, MMP-9, MMP-13 and MMP-14 genes after PGG treatment (1, 2.5, 5,10 and 20 μM). MMP-2 gene expression is significantly decreased with PGG at 5 and 10 μM (p < 0.0001, two-way ANOVA/Bonferroni) (n = 4). MMP-9 expression was not significantly different to the non-treated group (NT) at any PGG dose (p > 0.05, n = 4) (two-way ANOVA, Bonferroni). MMP-13 expression is decreased when Cal-27 cells are treated with 10 or 20 μM PGG (p < 0.05 and p < 0.001, respectively, two-way ANOVA/Bonferroni). MMP-13 gene expression is decreased when Cal-27 cells are treated with 10 or 20 μM PGG (p < 0.05 and p < 0.001, respectively, two-way ANOVA/Bonferroni) (p < 0.0001, two-way ANOVA) (n = 4). Housekeeping gene used was GAPDH
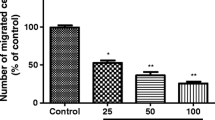
1,2,3,4,6-Penta-O-galloyl-ß-d-glucose (PGG) decreases invasiveness of Cal 27 cells Graph shows number of cells invading through Matrigel in the transmembrane/Boyden cell chamber. Number of cells invading through Matrigel was significantly lower in wells treated with PGG at 0.5 and 1 μM (p < 0.001, n = 4) (one-way ANOVA/Dunnett) and 2.5, 5 and 10 μM p < 0.0001, n = 4) (one-way ANOVA/Dunnett)
Protein extraction
During the protein extraction, media were collected, flash frozen and saved for BCA, Western Blot and ELISA analysis. Cells were washed with cold PBS and lysed with RIPA buffer (150 mM NaCl, 50 mM Tris, 1% Sodium deoxycholate, 1% Triton X-100 and 0.1% SDS) and collected with a cell scraper and centrifuged at 14,000 rpm for 30 min. The supernatant was collected and assayed for protein concentration using a BCA Assay (Cat# 23225, ThermoFisher Scientific). Samples were mixed with 4X SDS Loading buffer (40% Glycerol, 8% SDS, 200 mM Tris–HCl, 400 mM Dithiothreitol, 0.005% bromophenol blue), heated to 95˚C, and then frozen for further analysis.
Gel electrophoresis
12% Sodium dodecyl sulfate (SDS)—polyacrylamide gels were prepared by standard methods, loading 30 µg of protein per lane and electrophoresed for 1 h at 150 V.
Western blotting
Proteins from gels transferred to 0.45 µm nitrocellulose paper (Cat# 1620115, Bio-Rad, CA, USA) were then blocked with odyssey blocking buffer (Cat# 927-40000, LI-COR Biosciences, NE, USA). Primary antibodies were diluted in blocking solution containing 0.1% Tween and incubated overnight at 4 °C with monoclonal antibodies to MMP-1, MMP-3 (Cat# MAB901 and MAB 513; R&D Systems, Minneapolis, MN), MMP-2, MMP-9, Stat3, pStat3 (Cat# 13132, 13667, 12640, and 9145S Cell Signaling Technology, Danvers, MA, USA), MMP-8 and MMP-13 (Cat# ab53017 and ab39012; Abcam, Cambridge, MA, USA), and MMP-14 (Cat# AB8345; EMD Millipore, Billerica, MA, USA). Blots were normalized by probing the membranes with Histone H3 (Cat# 4499; Cell Signaling Technology, Beverly, MA, USA).
Secondary antibody incubation was performed in a blocking solution of 0.2% Tween with IRDye 800CW Goat anti-Rabbit IgG and IRDye® 680RD Donkey anti-Mouse IgG (Cat# 925-32211 and 925-68072; 1:10,000, LI-COR Biosciences, Lincoln, NE, USA). The proteins were detected and visualized by fluorescence using the LI-COR Odyssey Classic Infrared Imaging system (LI-COR Biosciences, Lincoln, NE, USA). Densitometry analysis of specific bands was performed with the Image Studio software provided by LI-COR Biosciences and the images analyzed using ImageStudio (LI-COR Biosciences). Statistical analysis, including one-way ANOVA with Dunnett’s Multiple Comparison Test and Bonferroni, was done using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). p values were calculated using the unpaired two-sided Student’s t test to compare groups, with statistical significance set at p < 0.05.
RNA preparation from tissue culture, reverse transcription and RT2-qPCR
After washing the tissue culture with 1× phosphate buffered saline (PBS), the cells were lysed, and the RNA purified using a Qiagen RNeasy mini kit (Cat# 74104) using the manufacturer's recommended protocol. All samples were treated with Qiagen DNase (Cat# 79254). One microgram of RNA was used for reverse transcription and subsequent SYBR® Green real time PCR for the genes of interest as previously described. Reverse transcription kits (Cat #330401) and SYBR Green real- time PCR master mixes (Cat# 330523) were from Qiagen (Louisville, KY, USA).
Primers and probes
The following primers and probes were used: Human MMP-2; MMP2 (Cat# PPH00151B), Human MMP-9; MMP9 (Cat#PPH00152E), Human MMP-13; MMP13 (Cat# PPH00121B), Human MMP-14; MMP14 (Cat# PPH00198C), and Human glyceraldehyde 3-phosphate dehydrogenase; GAPDH (Cat# PPH00150F).
Real time quantitative PCR was performed using an Applied Biosciences StepOne plus instrument and analyzed with StepOne software v2.3. The relative amounts of transcripts from each gene were normalized to reference gene GAPDH and calculated as follows: ∆∆CT = the average ∆CT of sample B—the average ∆CT of sample B, and their fold difference = 2−∆∆CT as previously described65.
Cell migration assays
Transmembrane/Boyden assay plates with Matrigel were purchased from Corning (Cat# 354480, NY, USA). DMEM with 10% FBS was used as a chemoattractant in the lower chamber while the cells in the upper chamber were cultured in DMEM with 1% FBS. Wells were prepared with different concentrations of PGG (0–10 µM) and left to incubate for 24 h. After 24 h media has discarded and the cells invading the Matrigel were counted according to established protocols [32].
Results
PGG decreased MMP-2, -9 and -13 secretion in Cal 27 cells
In the Cal 27 cell line, MMP-2 was released into the pericellular space and activated by MMP-14. Subsequently, MMP-2 and MMP-14 activate MMP-13. MMP-9 requires different molecules for activation. After incubating Cal-27 cells with PGG at different concentrations (1, 2.5, 5 and 10 μM), Cal-27 cells showed a dose-dependent decline of MMP-2 and MMP-13 release and at the 10 μM concentration the secretion was significantly lower than the non-treated cell group (Fig. 1a, c) MMP-9 secretion also decreased in a dose-dependent manner, becoming significantly lower than the non-treated group when PGG concentration reached 5 μM (Fig. 1b).
PGG decreases phosphorylation of Stat3
Our results demonstrated a decrease of Stat3 phosphorylation in the presence of varying concentrations of the small molecule PGG (Fig. 2). Results revealed an increase in total Stat3 when the cells were treated with PGG at varying concentrations. Despite a higher concentration of Stat3 present as substrate for phosphorylation to pStat3, PGG prevented the activation of this proinflammatory signal transducer.
PGG decreased the expression of MMP-2, MMP-13, and MMP-14 in a dose-dependent manner
Our data revealed that treatment with PGG at 10–20 µM reduces MMP-2, MMP-13, and MMP-14 expression when compared with non-treated Cal-27 (Fig. 3). MMP-9 gene expression did not significantly decrease with PGG, despite a decrease in the protein secretion with concentrations of PGG 5 µM and higher.
PGG reduces invasion of Cal 27 cells in a Matrigel transwell assay
Figure 4 illustrates the effects of PGG on the invasion of Cal 27 cells in vitro using Matrigel-coated transwells assay (Fig. 4). The data showed a significant decline in the percentage of cells invading the membrane after 24 h treatment when compared with the untreated control. The inhibitory effect is evident even with 0.5 µM PGG, with a maximum effect at 10 µM PGG (44.5% and 22.3%, respectively, Fig. 4).
Discussion
Despite having the largely preventable risk factors of tobacco and alcohol use, oral squamous cell cancer remains a serious, growing public health problem. Despite research seeking new treatment alternatives [33], survival rates have failed to significantly improve over the past decade. Even the presence of a single metastatic cervical lymph node drops OCSS survival rates by 50%, highlighting the potential benefit of therapy targeted at reducing tumor spread [34].
Previous research demonstrated that Stat3, a member of the JAK/STAT pathway, is constitutively expressed in many human cancers making it a suitable molecular target for anti-cancer therapy [35]. Human prostate cancer cell lines, DU145, PC3, and LNCaP, constitutively activate Stat3, promoting metastatic spread of cancer cells. In the human breast cancer cell line MCF-7, Stat3 is required for MMP-9 and MMP-1 activation, and results in cells with an enhanced migration rate [36]. The K-1735 melanoma system demonstrated that highly metastatic tumor cells expressed elevated MMP-2 mRNA and enzymatic activity. Moreover, a concomitantly elevated Stat3 activity was detected in these metastatic tumor cells that overexpress MMP-2. Stat3 inhibition in these cancer cells resulted in significant suppression of cell growth and cell invasiveness [37].
Consistent with research findings for other tumors, our results indicate that OSCC also exhibits persistent tyrosine phosphorylation of Stat3, stimulating cell proliferation and increasing differentiation, and migration [38]. OSCC also express the interleukin-6 receptor (IL6R), suggesting that PGG may block the inflammatory stimuli from Interleukin-6 (IL-6) derived from the tumor microenvironment [39]. Our findings showed that the small molecule inhibitor PGG can decrease MMP secretion and invasiveness in vitro through a Stat3 mechanism in Cal 27 cells. These results indicate that Cal 27 cells constitutively secrete MMP-2, -9, and -13. More importantly, they demonstrate that PGG inhibits the production of these MMPs in a dose-dependent manner. The Stat3/pStat3 effect is further reflected by decreases in MMP gene expression, accounting at least partly for the protein expression data for MMP-2, -9, and -13.
Understanding the complexity of Stat3 signaling and cross-talk between other significant molecules may offer strategies for developing novel pharmacologic therapies for cancer treatment and prevention. Despite the promise of MMP inhibition as a cancer treatment, clinical trials have been largely disappointing. While our findings suggest that PPG is a promising anti-cancer agent, further studies are needed to determine the effects of PGG on cell death and autophagy in a broader range of OSCC cell lines, as well as to elucidate other molecules that may play a role in initiating molecular signaling.
More studies are also needed to establish the pharmacokinetics of PGG and the feasibility of oral administration. Early pharmacokinetic studies for PGG show a delay in plasma C50 times with hard to detect plasmatic levels even at high oral doses [40]. Despite poor oral bioavailability, PGG binding to carrier molecules, such as albumin or lecithin could help prevent the rapid clearance of the small molecule PGG [40] and allow for adequate oral dosing. Previous research in a mice model of breast showed that oral administration of PGG is effective at decreasing metastasis, making it a promising alternative worthy of further studies [41].
Other studies indicate that small molecule inhibitors appear poised to become effective alternatives to suppress migration and invasion of oral cancer. For example, curcumin has shown to curtail the proliferation of Cal 27 cells by inhibiting Stat3 phosphorylation, IL-6 activity and to potentiate cisplatin through the inhibition of NF-B and its related proteins [17]. Our study indicates that PPG is another small molecule inhibitor that offers promise as an effective treatment for OCSS.
Conclusion
This study indicates that PPG is a small molecule that reduces the expression of matrix metalloproteinases and inhibits the metastatic spread of the Cal-27 squamous cell carcinoma cell line. By controlling the expression of molecules responsible for metastasis, PPG may offer a new therapeutic option for treating oral squamous cell carcinoma. Further exploration of PGG is needed in additional oral cancer cell lines to fully characterize anti-invasiveness potential. Considering that the tumor microenvironment exerts a significant effect over cancer cells, co-cultures of cancer associated fibroblasts and OSCC cell lines such as Cal-27, BHY, HSC-3, and HN would be essential to determine the actual usefulness of PGG as a therapeutic adjuvant in the treatment of OCSS.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Seoane J, Alvarez-Novoa P, Gomez I, Takkouche B, Diz P, Warnakulasiruya S, Seoane-Romero JM, Varela-Centelles P (2016) Early oral cancer diagnosis: the Aarhus statement perspective. A systematic review and meta-analysis. Head Neck 38:E2182–E2189. https://doi.org/10.1002/hed.24050
Rivera C, Venegas B (2014) Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett 8:7–11
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Rosenthal EL, Zhang W, Talbert M, Raisch KP, Peters GE (2005) Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote fibroblast-mediated type I collagen degradation in vitro. Mol Cancer Res 3:195–202. https://doi.org/10.1158/1541-7786.MCR-04-0203
Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, Tuluc M (2014) Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol 41:217–234. https://doi.org/10.1053/j.seminoncol.2014.03.003
Ma Y-S, Hsiao Y-P, Lin J-H, Hsu S-C, Chueh F-S, Weng S-W, Lai K-C, Lin J-G, Chung J-G (2015) Crude extract of Rheum palmatum L inhibits migration and invasion of LS1034 human colon cancer cells acts through the inhibition of matrix metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol 30:852–863. https://doi.org/10.1002/tox.21962
Dufour A, Overall CM (2013) Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci 34:233–242. https://doi.org/10.1016/j.tips.2013.02.004
Merdad A, Karim S, Schulten H-J, Dallol A, Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH (2014) Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res 34:1355–1366
Sharma M, Sah P, Sharma SS, Radhakrishnan R (2013) Molecular changes in invasive front of oral cancer. J Oral Maxillofac Pathol JOMFP 17:240–247. https://doi.org/10.4103/0973-029X.119740
Tamamura R, Nagatsuka H, Siar CH, Katase N, Naito I, Sado Y, Nagai N (2013) Comparative analysis of basal lamina type IV collagen α chains, matrix metalloproteinases-2 and -9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem 115:113–119. https://doi.org/10.1016/j.acthis.2012.05.001
Zhang X, Chen CT, Bhargava M, Torzilli PA (2012) A comparative study of fibronectin cleavage by MMP-1, -3, -13, and -14. Cartilage 3:267–277. https://doi.org/10.1177/1947603511435273
Thomas GT, Lewis MP, Speight PM (1999) Matrix metalloproteinases and oral cancer. Oral Oncol 35:227–233. https://doi.org/10.1016/S1368-8375(99)00004-4
Tsai S-C, Tsai M-H, Chiu C-F, Lu C-C, Kuo S-C, Chang N-W, Yang J-S (2016) AMPK-dependent signaling modulates the suppression of invasion and migration by fenofibrate in CAL 27 oral cancer cells through NF-κB pathway. Environ Toxicol 31:866–876. https://doi.org/10.1002/tox.22097
Fan M-J, Wang I-C, Hsiao Y-T, Lin H-Y, Tang N-Y, Hung T-C, Quan C, Lien J-C, Chung J-G (2015) Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing MMPs and NF-κB expressions in human oral cancer CAL 27 cells. Nutr Cancer 67:327–338. https://doi.org/10.1080/01635581.2015.990576
Huang H-W, Chung Y-A, Chang H-S, Tang J-Y, Chen I-S, Chang H-W (2014) Antiproliferative effects of methanolic extracts of Cryptocarya concinna Hance roots on oral cancer Ca9-22 and CAL 27 cell lines involving apoptosis, ROS induction, and mitochondrial depolarization. Sci World J 2014:e180462. https://doi.org/10.1155/2014/180462
Liao S, Xia J, Chen Z, Zhang S, Ahmad A, Miele L, Sarkar FH, Wang Z (2011) Inhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-κB signaling pathways. J Cell Biochem 112:1055–1065. https://doi.org/10.1002/jcb.23019
Mishev G, Deliverska E, Hlushchuk R, Velinov N, Aebersold D, Weinstein F, Djonov V (2014) Prognostic value of matrix metalloproteinases in oral squamous cell carcinoma. Biotechnol Biotechnol Equip 28:1138–1149. https://doi.org/10.1080/13102818.2014.967510
Pego ER, Fernández I, Núñez MJ (2018) Molecular basis of the effect of MMP-9 on the prostate bone metastasis: a review. Urol Oncol 36:272–282. https://doi.org/10.1016/j.urolonc.2018.03.009
Turpeenniemi-Hujanen T (2005) Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 87:287–297. https://doi.org/10.1016/j.biochi.2005.01.014
Jacob A, Prekeris R (2015) The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2015.00004
Cathcart J, Pulkoski-Gross A, Cao J (2015) Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis 2:26–34. https://doi.org/10.1016/j.gendis.2014.12.002
Łukaszewicz-Zając M, Mroczko B, Szmitkowski M (2011) Gastric cancer—the role of matrix metalloproteinases in tumor progression. Clin Chim Acta 412:1725–1730. https://doi.org/10.1016/j.cca.2011.06.003
Deryugina EI, Quigley JP (2015) Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol 44–46:94–112. https://doi.org/10.1016/j.matbio.2015.04.004
Che Y-L, Luo S-J, Li G, Cheng M, Gao Y-M, Li X-M, Dai J-M, He H, Wang J, Peng H-J, Zhang Y, Li W-Y, Wang H, Liu B, Linghu H (2015) The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett 359:241–249. https://doi.org/10.1016/j.canlet.2015.01.019
Lin VC-H, Kuo P-T, Lin Y-C, Chen Y, Hseu Y-C, Yang H-L, Kao J-Y, Ho C-T, Way T-D (2014) Penta-O-galloyl-β-d-glucose suppresses EGF-induced eIF3i expression through inhibition of the PI3K/AKT/mTOR pathway in prostate cancer cells. J Agric Food Chem 62:8990–8996. https://doi.org/10.1021/jf502447e
Feldman KS, Sahasrabudhe K, Lawlor MD, Wilson SL, Lang CH, Scheuchenzuber WJ (2001) In vitro and in vivo inhibition of LPS-stimulated tumor necrosis factor-α secretion by the gallotannin β-d-pentagalloylglucose. Bioorg Med Chem Lett 11:1813–1815. https://doi.org/10.1016/S0960-894X(01)00332-8
Ho L-L, Chen W-J, Lin-Shiau S-Y, Lin J-K (2002) Penta-O-galloyl-β-d-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur J Pharmacol 453:149–158. https://doi.org/10.1016/S0014-2999(02)02340-3
Kant R, Yen C-H, Lu C-K, Lin Y-C, Li J-H, Chen Y-MA (2016) Identification of 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranoside as a glycine N-methyltransferase enhancer by high-throughput screening of natural products inhibits hepatocellular carcinoma. Int J Mol Sci. https://doi.org/10.3390/ijms17050669
Kuo PT, Lin TP, Liu LC, Huang CH, Lin JK, Kao JY, Way TD (2009) Penta-O-galloyl-β-d-glucose suppresses prostate cancer bone metastasis by transcriptionally repressing EGF-induced MMP-9 expression. J Agric Food Chem. https://doi.org/10.1021/jf803725h (ACS Publications)
Parasaram V, Nosoudi N, Chowdhury A, Vyavahare N (2018) Pentagalloyl glucose increases elastin deposition, decreases reactive oxygen species and matrix metalloproteinase activity in pulmonary fibroblasts under inflammatory conditions. Biochem Biophys Res Commun 499:24–29. https://doi.org/10.1016/j.bbrc.2018.03.100
Hall DMS, Brooks SA (2014) In vitro invasion assay using MatrigelTM: a reconstituted basement membrane preparation. In: Dwek M, Schumacher U, Brooks SA (eds) Metastasis research protocols. Springer, New York, pp 1–11
Rivera C (2015) Essentials of oral cancer. Int J Clin Exp Pathol 8:11884–11894
Dong F, Tao C, Wu J, Su Y, Wang Y, Wang Y, Guo C, Lyu P (2018) Detection of cervical lymph node metastasis from oral cavity cancer using a non-radiating, noninvasive digital infrared thermal imaging system. Sci Rep 8:7219. https://doi.org/10.1038/s41598-018-24195-4
Turkson J, Jove R (2000) STAT proteins: novel molecular targets for cancer drug discovery. Oncogene 19:6613–6626. https://doi.org/10.1038/sj.onc.1204086
Lee JH, Kim JE, Kim BG, Han HH, Kang S, Cho NH (2016) STAT3-induced WDR1 overexpression promotes breast cancer cell migration. Cell Signal 28:1753–1760. https://doi.org/10.1016/j.cellsig.2016.08.006
Xie T, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S (2004) Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23:3550–3560. https://doi.org/10.1038/sj.onc.1207383
Gkouveris I, Nikitakis N, Sklavounou A (2018) p38 expression and modulation of STAT3 signaling in oral cancer. Pathol Oncol Res. https://doi.org/10.1007/s12253-018-0405-9
Gasche JA, Hoffmann J, Boland CR, Goel A (2011) Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer 129:1053–1063. https://doi.org/10.1002/ijc.25764
Jiamboonsri P, Pithayanukul P, Bavovada R, Leanpolchareanchai J, Yin T, Gao S, Hu M (2015) Factors influencing oral bioavailability of Thai mango seed kernel extract and its key phenolic principles. Molecules 20:21254–21273. https://doi.org/10.3390/molecules201219759
Lee H-J, Seo N-J, Jeong S-J, Park Y, Jung D-B, Koh W, Lee H-J, Lee E-O, Ahn KS, Ahn KS, Lü J, Kim S-H (2011) Oral administration of penta-O-galloyl-β-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 32:804–811. https://doi.org/10.1093/carcin/bgr015
Acknowledgements
The authors wish to thank Roseman University of Health Sciences, College of Dental Medicine, South Jordan, UT for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. No animals or human subjects were used in any of the experiments described in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matias, C., Bordieri, T., Roberts, D. et al. Small molecule inhibition of matrix metalloproteinases as a potential therapeutic for metastatic activity in squamous cell carcinoma. Oral Cancer 3, 1–8 (2019). https://doi.org/10.1007/s41548-019-00017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41548-019-00017-7