Abstract
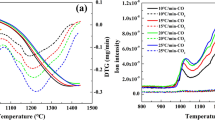
Iron and steel have a significant economic and application importance, therefore the reduction of iron oxides is of interest to many researchers. There is a high-energy demand for steel and iron making industries, and it is crucial to address the kinetics of reactions on natural gas as a source of energy and reducing agent for most steel companies. In this thermogravimetric study, the reaction kinetics of Fe3O4 fine particles by using CH4 reduction has been investigated at temperatures ranging from 800 to 1100 °C which have been compared with the results obtained from concentrate reduction by H2 and CO. Different models have been assessed to obtain the governing mechanism and kinetics parameters of this reaction. Unlike H2 and CO reducing agents, the sintering phenomenon increases the rate of CH4 reduction at temperatures more than 900 °C. Dependency of the reaction rate on the amount of Fe3O4 is classified as first-order reaction and this dependency on their particle size is a power function with a − 1.871 power. The reaction rate also is considered a power function of CH4 concentration with − 4.236 power. The reaction activation energy estimated equal to142.5 kJ mol−1.
















Similar content being viewed by others
Data availability
All the experimental data and numerical method data have been presented in the paper. Nevertheless, any required data will be available by email the corresponding author.
References
Abolpour B, Afsahi MM, Azizkarimi M (2018) Reduction of magnetite concentrate particles by carbon monoxide. Miner Process Extr Metall Trans Instit Min Metall 127(1):29–39
Abolpour B, Afsahi MM, Azizkarimi M (2021) Hydrogen reduction of magnetite concentrate particles. Miner Process Extr Metall. https://doi.org/10.1080/25726641.2018.1521576
Ale Ebrahim H, Jamshidi E (2001) Kinetic study of zinc oxide reduction by methane. Chem Eng Res Des 79(1):62–70
Alizadeh R, Jamshidi E, Ale Ebrahim H (2007) Kinetic study of nickel oxide reduction by methane. Chem Eng Technol 30(8):1123–1128
Barrett D (1972) Partial oxidation of methane using iron oxide as donor. Ind Eng Chem Process Des Dev 11(3):415–420
Brown WE, Dollimore D, Galwey AK (1980) Reactions in the solid state. In: Bamford CH, Tipper CHF (eds) Comprehensive chemical kinetics, vol 22. Elsevier, Amsterdam
Chen F, Mohasseb Y, Zhang S, Sohn HY (2015a) Kinetics of the reduction of hematite concentrate particles by carbon monoxide relevant to a novel flash ironmaking process. Metall Mater Trans B. https://doi.org/10.1007/s11663-015-0345-7
Chen F, Mohasseb Y, Jiang T, Sohn HY (2015b) Hydrogen reduction kinetics of hematite concentrate particles relevant to a novel flash ironmaking process. Metall Mater Trans B 46:1133–1145
Choi ME (2010) Suspension hydrogen reduction of iron ore concentrate, a dissertation for the degree of Doctor of Philosophy, Department of Metallurgical Engineering, The University of Utah, USA
El-Rahaiby SK, Rao YK (1979) The kinetics of reduction of iron oxides at moderate temperatures. Metal Trans B 10:258–264
Keshavarzzadeh M, Zahedi R, Eskandarpanah R, Qezelbigloo S, Gitifar S, Noudeh Farahani O, Mirzaei AM (2023) Estimation of NOx pollutants in a spark engine fueled by mixed methane and hydrogen using neural networks and genetic algorithm. Heliyon 9(4):e15304
Khoshandam B, Jamshidi E, Kumar RV (2004) Reduction of cobalt oxide with methane. Metall Mater Trans B 35(5):825–828
Levenspiel O (1999) Chemical reaction engineering, 3rd edn. Wiely, New York
Moon J, Sahajwalla V (2006) Investigation into the role of the boudouard reaction in self-reducing iron oxide and carbon briquettes. Metall Mater Trans B 37(2):215–221
Nakagawa H, Maeda T, Nishioka K, Shimizu M (2005) Ironmaking - rapid reduction of fine iron ore transported with CH4 gas. Tetsu-to-Hagane 91(6):521–527
Ostrovski O, Zhang G (2006) Reduction and carburization of metal oxides by methane-containing gas. AIChE J 52(1):300–310
Pourghahramani P, Forssberg E (2007) Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods. Thermochemica Acta 454(2):69–77
Roine A (2006) HSC Chemistry 6.0, Chemical Reaction and Equilibrium Software with Extensive Termochemical Database and Flowsheet Simulation, Outokumpu Research Oy Information Center
Takeuchi N, Nomura Y, Ohono K, Maeda T, Nishioka K, Shimizu M (2007) Kinetic analysis of spherical wostite reduction transported with CH4 gas. ISIJ Int 47(3):386–391
Vyazovkin S, Wight CA (1999) Model free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340:53–68
Wang H, Choi ME, Sohn HY (2011) Intrinsic hydrogen reduction kinetics of magnetite concentrate particles relevant to a novel green ironmaking technology, 2nd International Symposium on High-Temperature Metallurgical Processin, San Diego, USA, 2011, pp. 3–10
Zahedi R, Ayazi M, Aslani A (2022) Comparison of amine adsorbents and strong hydroxides soluble for direct air CO2 capture by life cycle assessment method. Environ Technol Innov 28:102854
Zahedi R, Shaghaghi A, TaghiTahooneh M, Ahmadi A (2023) Numerical simulation of combustion of sulfide-biomass concentrate ingredients and contaminants in copper furnace smelting. Future Energy 2(1):1–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abolpour, B., Afsahi, M.M. Gas–Solid Reaction Kinetic and Mechanism Between Fe3O4 and CH4. Trans Indian Natl. Acad. Eng. 9, 199–210 (2024). https://doi.org/10.1007/s41403-023-00445-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-023-00445-4