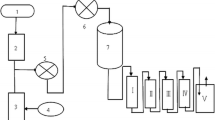
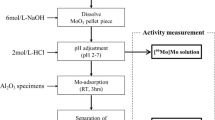
Abstract
Radioiodine-131 is one of the pernicious radionuclides released during nuclear accidents, as its radioactivity can potentially affect public health and safety. To prevent radioiodine-131 from being released into the environment, the use of adsorbents that are highly efficient at high temperatures is significantly important. The radioactive gas from the nuclear core in an accident, such as the Fukushima nuclear accident, is usually released occurs under high-temperature conditions. Therefore, in this study, a 10 wt% silver phosphate-loaded alumina (Ag3PO4/Al2O3) adsorbent was prepared. Further, its performance toward radioiodine adsorption was tested at high temperatures up to 750 °C, using Al2O3 and traditional 10 wt% Ag/Al2O3 adsorbent as controls. The results of the iodine adsorption test indicated that the 10 wt% Ag3PO4/Al2O3 adsorbent showed a higher decontamination factor than did the 10 wt% Ag/Al2O3 adsorbent by two orders of magnitude at 650 and 750 °C. Results of the iodine desorption test revealed that the new adsorbent could be effectively used at 750 °C. The characteristic powder X-ray diffraction, nitrogen adsorption–desorption isotherm, X-ray photoelectron spectroscopy, and thermogravimetric analysis–differential scanning calorimetry data indicated that the enhanced adsorption ability at high temperatures was attributed to the formation of a solid solution between silver iodide and Ag3PO4.








Similar content being viewed by others
References
H.A. Handford, S.D. Mayes, R.E. Mattison et al., Child and parent reaction to the Three Mile Island nuclear accident. J Am Acad Child Psychiatry 25(3), 346–356 (1986). https://doi.org/10.1016/S0002-7138(09)60256-9
M. Hatch, E. Ron, A. Bouville et al., The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Epidemiol. Rev. 27(1), 56–66 (2005). https://doi.org/10.1093/epirev/mxi012
J.E. Ten Hoeve, M.Z. Jacobson, Worldwide health effects of the Fukushima Daiichi nuclear accident. Energy Environ. Sci. 5(9), 8743–8757 (2012). https://doi.org/10.1039/C2EE22019A
B.B.F. Wittneben, The impact of the Fukushima nuclear accident on European energy policy. Environ. Sci. Policy 15(1), 1–3 (2012). https://doi.org/10.1016/j.envsci.2011.09.002
L.S. Lebel, R.S. Dickson, G.A. Glowa, Radioiodine in the atmosphere after the Fukushima Dai-ichi nuclear accident. J. Environ. Radioact. 151, 82–93 (2016). https://doi.org/10.1016/j.jenvrad.2015.06.001
W.M. Haynes (ed.), CRC Handbook of Chemistry and Physics (CRC Press, Boca Raton, 2014)
X. Hou, V. Hansen, A. Aldahan et al., A review on speciation of iodine-129 in the environmental and biological samples. Anal. Chim. Acta 632(2), 181–196 (2009). https://doi.org/10.1016/j.aca.2008.11.013
Y. Nitta, M. Hoshi, K. Kamiya, Effects of radioactive iodine (131I) on the thyroid of newborn, pubertal and adult rats. Paper presented at the 17th World Congress of the International Federation of Otorhinolaryngological Societies, Cairo, September 2002
M. Ali, C. Yan, Z. Sun et al., Study of iodine removal efficiency in self-priming venturi scrubber. Ann. Nucl. Eng. 57, 263–268 (2013). https://doi.org/10.1016/j.anucene.2013.02.014
I. Younus, M.S. Yim, Out-containment mitigation of gaseous iodine by alkaline spray in severe accident situation. Prog. Nucl. Energy 83, 167–176 (2015). https://doi.org/10.1016/j.pnucene.2015.03.012
T.M. Nenoff, M.A. Rodriguez, N.R. Soelberg et al., Silver-mordenite for radiologic gas capture from complex streams: dual catalytic CH3I decomposition and I confinement. Microporous Mesoporous Mater. 200, 297–303 (2014). https://doi.org/10.1016/j.micromeso.2014.04.041
K.W. Chapman, P.J. Chupas, T.M. Nenoff, Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 132(26), 8897–8899 (2010). https://doi.org/10.1021/ja103110y
L. Wu, J.A. Sawada, D.B. Kuznicki et al., Iodine adsorption on silver-exchanged titania-derived adsorbents. J. Radioanal. Nucl. Chem. 302(1), 527–532 (2014). https://doi.org/10.1007/s10967-014-3252-5
Q. Cheng, Z. Li, T. Chu, Adsorption of gaseous iodine-131 at high temperatures by silver impregnated alumina. Nucl. Sci. Tech. 26(04), 43–47 (2015). https://doi.org/10.13538/j.1001-8042/nst.26.040303
M. Outokesh, A. Saket, S.J. Ahmadi et al., Comparative study on adsorption of iodine vapor by silica-supported Cu nanoparticles and micronized copper. Ind. Eng. Chem. Res. 51(47), 15315–15323 (2012). https://doi.org/10.1021/ie301263j
Y. Watanabe, T. Ikoma, H. Yamada et al., Novel long-term immobilization method for radioactive iodine-129 using a zeolite/apatite composite sintered body. ACS Appl Mat Interfaces. 1(7), 1579–1584 (2009). https://doi.org/10.1021/am900251m
J. Matyas, G. Fryxell, B. Busche et al., in Functionalised silica aerogels: advanced materials to capture and immobilise radioactive iodine, ed. by Yutai Katoh, M.F. Kevin, H. Lin et al., Ceramic engineering and science proceedings, 735 ceramic place Westerville OH, November 2011, vol. 32 (American Ceramic Society, Inc., 2011), pp. 23–32
H. Sun, P. La, Z. Zhu et al., Capture and reversible storage of volatile iodine by porous carbon with high capacity. J. Mater. Sci. 50(22), 7326–7332 (2015). https://doi.org/10.1007/s10853-015-9289-1
S. Hirunpraditkoon, P. Srinophakun, N. Sombun et al., Synthesis of activated carbon from jatropha seed coat and application to adsorption of iodine and methylene blue. Chem. Eng. Commun. 202(1), 32–47 (2015). https://doi.org/10.1080/00986445.2013.828611
B.J. Riley, D.A. Pierce, J. Chun et al., Polyacrylonitrile-chalcogel hybrid sorbents for radioiodine capture. Environ. Sci. Technol. 48(10), 5832–5839 (2014). https://doi.org/10.1021/es405807w
B.J. Riley, J. Chun, J.V. Ryan et al., Chalcogen-based aerogels as a multifunctional platform for remediation of radioactive iodine. RSC Advances. 1(9), 1704–1715 (2011). https://doi.org/10.1039/C1RA00351H
S.U. Nandanwar, K. Coldsnow, V. Utgikar et al., Capture of harmful radioactive contaminants from off-gas stream using porous solid sorbents for clean environment—a review. Chem. Eng. J. 306, 369–381 (2016). https://doi.org/10.1016/j.cej.2016.07.073
B.J. Riley, J.D. Vienna, D.M. Strachan et al., Materials and processes for the effective capture and immobilization of radioiodine: a review. J. Nucl. Mater. 470, 307–326 (2016). https://doi.org/10.1016/j.jnucmat.2015.11.038
Q. Cheng, W. Yang, Z. Li et al., Adsorption of gaseous radioactive iodine by Ag/13X zeolite at high temperatures. J. Radioanal. Nucl. Chem. 303(3), 1883–1889 (2015). https://doi.org/10.1007/s10967-014-3736-3
T. Takahashi, S. Ikeda, O. Yamamoto, Solid-State Ionics—solids with high ionic conductivity in the systems silver iodide-silver oxyacid salts. J. Electrochem. Soc. 119(4), 477–482 (1972). https://doi.org/10.1149/1.2404235
N. Koga, S. Yamada, T. Kimura, Thermal decomposition of silver carbonate: phenomenology and physicogeometrical kinetics. J. Phys. Chem. C 117(1), 326–336 (2012). https://doi.org/10.1021/jp309655s
Y. Liu, L. Fang, H. Lu et al., One-pot pyridine-assisted synthesis of visible-light-driven photocatalyst Ag/Ag3PO4. Appl. Catal. B 115, 245–252 (2012). https://doi.org/10.1016/j.apcatb.2011.12.038
Y. Nan, L.L. Tavlarides, D.W. DePaoli, Adsorption of iodine on hydrogen-reduced silver-exchanged mordenite: experiments and modeling. AlChE J. 63(3), 1024–1035 (2017). https://doi.org/10.1002/aic.15432
V.I. Yakerson, A.M. Rubinshtein, Thermal desorption of water from the surface of aluminum oxide. Russ. Chem. Bull. 16(6), 1319–1321 (1967). https://doi.org/10.1007/BF00908303
M. Reháková, A. Sopková, K. Jesenák et al., Thermal analysis of the synthetic zeolite ZSM5 and its silver iodide form. J. Therm. Anal. Calorim. 50(3), 505–509 (1997). https://doi.org/10.1007/BF00908303
N.C. Hyatt, J.A. Hriljac, A. Choudhry et al., Zeolite-salt occlusion: a potential route for the immobilisation of iodine-129? Paper presented at MRS Proceedings, Cambridge University, Cambridge, 2003
S. Zhang, X. Gu, Y. Zhao et al., Effect of annealing temperature and time on structure, morphology and visible-light photocatalytic activities Ag3PO4 microparticles. Mater. Sci. Eng., B 201, 57–65 (2015). https://doi.org/10.1016/j.mseb.2015.08.005
N. Machida, S. Nishida, T. Shigematsu et al., Mechano chemical synthesis of a silver ion conductor in the system AgI–Ag3PO4. Solid State Ion. 136, 381–386 (2000). https://doi.org/10.1016/S0167-2738(00)00488-4
J.R. Casanova, J.C.L. Tonazzi, J.I. Franco, Interaction of I2 with Ag7I4PO4. Solid State Ion. 96(1), 107–111 (1997). https://doi.org/10.1016/S0167-2738(96)00618-2
V.K. Sarin (ed.), Comprehensive Hard Materials (Elsevier Ltd., New York, 2014)
R.T. Jubin, G.D. DelCul, B.D. Patton et al., Advanced fuel cycle initiative coupled end-to-end research, development, and demonstration project: integrated off-gas treatment system design and initial performance-9226. Paper presented at WM2009 conference, WM symposia, Phoenix AZ, March 1-5 2009
K.H. Lieser, Radiochemische Messung der Löslichkeit von Silberhalogeniden in Wasser und in Natriumhalogenidlösungen und die Komplexbildung der Silberhalogenide mit Halogenionen. Z. Anorg. Allg. Chem. 292(1–3), 97–113 (1957). https://doi.org/10.1002/zaac.19572920113
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Nos. 11575010 and 21201013).
Rights and permissions
About this article
Cite this article
Wang, X., Chu, TW. Formation of AgI/Ag3PO4 solid solution on alumina for enhancing radioactive iodine adsorption at high temperatures. NUCL SCI TECH 29, 64 (2018). https://doi.org/10.1007/s41365-018-0408-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-018-0408-y