Abstract
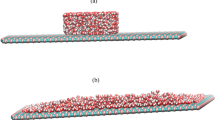
Water molecules could form a liquid droplet on the water monolayer on a specific solid surface, which has been referred to as “ordered water monolayer that does not completely wet water” at room temperature. In contrast to the water molecules, the family of alcohol molecules has the same OH polar head and various lengths of their hydrophobic nonpolar tail; the length of the hydrophobic tail can affect the hydrophobic effect. In this study, using molecular dynamics simulations, we investigated the wetting behaviors of methanol, ethanol, and propanol molecules adsorbed on a SiO2 surface. The results showed that the methanol, ethanol, and propanol molecules could form an ordered monolayer on the SiO2 surface and a droplet on top of this monolayer, with different contact angles. The differences in the contact angles were attributed to the differences in the interactions between the alcohol monolayer and droplet.







Similar content being viewed by others
References
C.K. Wu, L.J. Chen, Wetting behavior of mixtures of water and nonionic polyoxyethylene alcohol. Langmuir 21, 6883–6890 (2005). https://doi.org/10.1021/la050691i
S.J. Shao, P. Guo, L. Zhao et al., Ordered water monolayer on ionic model substrates studied by molecular dynamics simulations. Nucl. Sci. Tech. 25, 020502 (2014). https://doi.org/10.13538/j.1001-8042/nst.25.020502
G.L. Chang, D.G. Wang, Y.Y. Zhang et al., Ald-coated ultrathin al2o3 film on bivo4 nanoparticles for efficient pec water splitting. Nucl. Sci. Tech. 27, 108 (2016). https://doi.org/10.1007/s41365-016-0122-6
P.I. Girginova, A.L. Daniel-da-Silva, C.B. Lopes et al., Silica coated magnetite particles for magnetic removal of hg 2+ from water. J. Colloid Interface Sci. 345, 234–240 (2010). https://doi.org/10.1016/j.jcis.2010.01.087
H. Yang, R. Xu, X. Xue et al., Hybrid surfactant-templated mesoporous silica formed in ethanol and its application for heavy metal removal. J. Hazard. Mater. 152, 690–698 (2008). https://doi.org/10.1016/j.jhazmat.2007.07.060
Y. Sun, L. Duan, Z. Guo et al., An improved way to prepare superparamagnetic magnetite-silica core-shell nanoparticles for possible biological application. J. Magn. Magn. Mater. 285, 65–70 (2005). https://doi.org/10.1016/j.jmmm.2004.07.016
X. Ren, B. Zhou, C. Wang, Promoting effect of ethanol on dewetting transition in the confined region of melittin tetramer. Nucl. Sci. Tech. 23, 252–256 (2012). https://doi.org/10.13538/j.1001-8042/nst.23.252-256
P. Wang, R.B. Zhong, M. Yuan et al., Mercury (ii) detection by water-soluble photoluminescent ultra-small carbon dots synthesized from cherry tomatoes. Nucl. Sci. Tech. 27, 35 (2016). https://doi.org/10.1007/s41365-016-0038-1
W. Xu, Y.S. Tu, C.L. Wang et al., Water transport through t-shaped carbon nanotubes. Nucl. Sci. Tech. 22, 307–310 (2011). https://doi.org/10.13538/j.1001-8042/nst.22.307-310
J.J. Karnes, E.A. Gobrogge, R.A. Walker et al., Unusual structure and dynamics at silica/methanol and silica/ethanol interfaces: a molecular dynamics and nonlinear optical study. J. Phys. Chem. B 120, 1569–1578 (2015). https://doi.org/10.1021/acs.jpcb.5b07777
E.A. Gobrogge, R.A. Walker, Binary solvent organization at silica/liquid interfaces: preferential ordering in acetonitrile–methanol mixtures. J. Phys. Chem. Lett 5, 2688–2693 (2014). https://doi.org/10.1021/jz500906d
M.R. Brindza, R.A. Walker, Differentiating solvation mechanisms at polar solid/liquid interfaces. J. Am. Chem. Soc. 131, 6207–6214 (2009). https://doi.org/10.1021/ja810117f
A.R. Siler, R.A. Walker, Effects of solvent structure on interfacial polarity at strongly associating silica/alcohol interfaces. J. Phys. Chem. C 115, 9637–9643 (2011). https://doi.org/10.1021/jp201153z
T. Cheng, H. Sun, Adsorption of ethanol vapor on mica surface under different relative humidities: a molecular simulation study. J. Phys. Chem. C 116, 16436–16446 (2012). https://doi.org/10.1021/jp3020595
A. Phan, D.R. Cole, A. Striolo, Liquid ethanol simulated on crystalline alpha alumina. J. Phys. Chem. B 117, 3829–3840 (2013). https://doi.org/10.1021/jp312238d
H.L. Rossetto, J. Bowen, K. Kendall, Adhesion of alumina surfaces through confined water layers containing various molecules. Langmuir 28, 4648–4653 (2012). https://doi.org/10.1021/la2045064
I.S. Pasarín, M. Yang, N. Bovet et al., Molecular ordering of ethanol at the calcite surface. Langmuir 28, 2545–2550 (2012). https://doi.org/10.1021/la2021758
D. Wu, A. Navrotsky, Probing the energetics of organic–nanoparticle interactions of ethanol on calcite. Proc. Natl. Acad. Sci. USA. 112, 5314–5318 (2015). https://doi.org/10.1073/pnas.1505874112
J. Sung, G.A. Waychunas, Y.R. Shen, Surface-induced anisotropic orientations of interfacial ethanol molecules at air/sapphire(1\(\overline{1}\)02) and ethanol/sapphire(1\(\overline{1}\)02) interfaces. J. Phys. Chem. Lett 2, 1831–1835 (2011). https://doi.org/10.1021/jz2006397
C. Wang, H. Lu, Z. Wang et al., Stable liquid water droplet on a water monolayer formed at room temperature on ionic model substrates. Phys. Rev. Lett. 103, 137801 (2009). https://doi.org/10.1103/PhysRevLett.103.137801
C.L. Wang, H.P. Fang, “Ordered water monolayer that does not completely wet water” at room temperature and molecular-scale hydrophilicity (in chinese). Sci. China-Phys. Mech. Astron 46, 74–83 (2016). https://doi.org/10.1360/SSPMA2015-00603
C. Wang, Y. Yang, H. Fang, Recent advances on “ordered water monolayer that does not completely wet water” at room temperature. Sci. China-Phys. Mech. Astron 57, 802–809 (2014). https://doi.org/10.1007/s11433-014-5415-3
C. Qi, B. Zhou, C. Wang et al., A nonmonotonic dependence of the contact angles on the surface polarity for a model solid surface. Phys. Chem. Chem. Phys. 19, 6665–6670 (2017). https://doi.org/10.1039/c6cp08275k
P. Guo, Y. Tu, J. Yang et al., Water-cooh composite structure with enhanced hydrophobicity formed by water molecules embedded into carboxyl-terminated self-assembled monolayers. Phys. Rev. Lett. 115, 186101 (2015). https://doi.org/10.1103/PhysRevLett.115.186101
D.T. Limmer, A.P. Willard, P. Madden et al., Hydration of metal surfaces can be dynamically heterogeneous and hydrophobic. Proc. Natl. Acad. Sci. USA. 110, 4200–4205 (2013). https://doi.org/10.1073/pnas.1301596110
Z. Xu, Y. Gao, C. Wang et al., Nanoscale hydrophilicity on metal surfaces at room temperature: coupling lattice constants and crystal faces. J. Phys. Chem. C 119, 20409–20415 (2015). https://doi.org/10.1021/acs.jpcc.5b04237
J. Lützenkirchen, R. Zimmermann, T. Preočanin et al., An attempt to explain bimodal behaviour of the sapphire c-plane electrolyte interface. Adv. Colloid Interface Sci. 157, 61–74 (2010). https://doi.org/10.1016/j.cis.2010.03.003
B. Rotenberg, A.J. Patel, D. Chandler, Molecular explanation for why talc surfaces can be both hydrophilic and hydrophobic. J. Am. Chem. Soc. 133, 20521–20527 (2011). https://doi.org/10.1021/ja208687a
A. Phan, T.A. Ho, D. Cole et al., Molecular structure and dynamics in thin water films at metal oxide surfaces: magnesium, aluminum, and silicon oxide surfaces. J. Phys. Chem. C 116, 15962–15973 (2012). https://doi.org/10.1021/jp300679v
L. Zhao, C. Wang, J. Liu et al., Reversible state transition in nanoconfined aqueous solutions. Phys. Rev. Lett. 112, 078301 (2014). https://doi.org/10.1103/PhysRevLett.112.078301
L. Zhao, C.L. Wang, H.P. Fang et al., The gibbs-free-energy landscape for the solute association in nanoconfined aqueous solutions. Nucl. Sci. Tech. 26, 030504 (2015). https://doi.org/10.13538/j.1001-8042/nst.26.030504
C. Wang, L. Zhao, D. Zhang et al., Upright or flat orientations of the ethanol molecules on a surface with charge dipoles and the implication for wetting behavior. J. Phys. Chem. C 118, 1873–1878 (2014). https://doi.org/10.1021/jp4062016
X. Nie, J. Chen, N. Sheng et al., Effect of water molecules on nanoscale wetting behaviour of molecular ethanol on hydroxylated SiO2 substrate. Mol. Simul. 43, 1377–1384 (2017). https://doi.org/10.1080/08927022.2017.1353692
R.T. Cygan, J.-J. Liang, A.G. Kalinichev, Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 108, 1255–1266 (2004). https://doi.org/10.1021/jp0363287
X. Ren, B. Zhou, C. Wang, Water-induced ethanol dewetting transition. J. Chem. Phys. 137, 024703 (2012). https://doi.org/10.1063/1.4733719
X. Ren, C. Wang, B. Zhou et al., Ethanol promotes dewetting transition at low concentrations. Soft Matter 9, 4655–4660 (2013). https://doi.org/10.1039/C3SM00049D
W.L. Jorgensen, D.S.M. And, J. Tiradorives, Development and testing of the opls all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996). https://doi.org/10.1021/ja9621760
D. Van Der Spoel, E. Lindahl, B. Hess et al., Gromacs: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005). https://doi.org/10.1002/jcc.20291
C.-J. Shih, Q.H. Wang, S. Lin et al., Breakdown in the wetting transparency of graphene. Phys. Rev. Lett. 109, 176101 (2012). https://doi.org/10.1103/PhysRevLett.109.176101
G. Vazquez, E. Alvarez, J.M. Navaza, Surface tension of alcohol water + water from 20 to 50. Degree. C. J. Chem. Eng. Data 40, 611–614 (1995). https://doi.org/10.1021/je00019a016
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Natural Science Foundation of China (Nos. U1532260, 11674345, and 11504032), Key Research Program of Chinese Academy of Sciences (Nos. KJZD-EW-M03 and QYZDJ-SSW-SLH019), Youth Innovation Promotion Association CAS (Grant Number 2014233), Shanghai Supercomputer Center of China, Computer Network Information Center of Chinese Academy of Sciences, and Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase).
Rights and permissions
About this article
Cite this article
Nie, XC., Zhou, B., Wang, CL. et al. Wetting behaviors of methanol, ethanol, and propanol on hydroxylated SiO2 substrate. NUCL SCI TECH 29, 18 (2018). https://doi.org/10.1007/s41365-018-0364-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-018-0364-6