Abstract
Avocado (Persea americana Milll.) holds a pivotal position in global fruit crops, contributing significantly to the economies of tropical and subtropical regions. However, the rising incidence of diseases poses a substantial risk to avocado production. This comprehensive study investigated the disease landscape in Antalya, the largest avocado cultivation area in the Türkiye. A survey of 2537 avocado trees across 11 regions from 2020 to 2021 revealed alarming disease incidences, particularly in the eastern regions of Gazipasa and Alanya. Dieback, branch canker, anthracnose, and soil-borne root rot were identified as the primary diseases affecting tree canopies, twigs, and branches. Morphological and molecular analyzes unveiled a spectrum of pathogens, with Colletotrichum gloeosporioides dominating in the Mediterranean region. Notably, Phytophthora cinnamomi emerged as a severe threat, causing root rot and decline in avocado trees. Fusarium solani and Fusarium oxysporum, known for their association with tropical fruit crops, were identified in the western parts of Antalya. Additionally, we have detected Neofusicoccum parvum, Lasiodiplodia theobromae, and Neopestalotiopsis rosae in collected samples from avocado trees. The identified pathogens exhibited varying levels of severity in branch canker and anthracnose on avocado branches and leaves. Furthermore, pathogenicity evaluations shed light on the potential of these pathogens to induce severe symptoms, emphasizing the urgency for effective control measures. The exploration of cultural and biological control strategies are crucial for mitigating the impact of branch canker, dieback, and anthracnose diseases, ensuring the sustainability of avocado cultivation in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avocado stands as a pivotal fruit crop globally, thriving in tropical and subtropical regions. The Mediterranean region of Türkiye, boasting favorable climatic conditions, has emerged as a significant area for the cultivation of this exotic fruit. Notably, avocado has gained momentum in the Mediterranean regions of various countries, with Türkiye witnessing a remarkable surge in production. In the year 2019 alone, production reached 4209 tons, covering an expansive area of 949 hectares, and sustaining over 80,000 avocado trees.
However, various pathogens causing diseases in avocado production have resulted in a drastic reduction in avocado growth and productivity, ultimately leading to increased production costs (Ramírez-Gil et al. 2017). Foliar diseases, caused by airborne fungal pathogens such as Diplodia species and Dothiorella species (Botryosphaeriaceae: Ascomycota), along with many members of the Botryosphaeriaceae family, are associated with branch canker, dieback, and stem-end rot in avocados in various countries, resulting in significant economic losses (McDonald and Eskalen 2011; Valencia et al. 2019). However, the causal agent(s) of these diseases in the Mediterranean region of Türkiye are not known. Although some fungal species can survive as endophytes in symptomless tissue, the fungi in the Botryosphaeriaceae family are reported to be pathogenic on many host plants under stress conditions (Valencia et al. 2019; Wanjiku et al. 2020). Typical symptoms that develop on twigs, branches, and stems include shriveling, followed by brown to black rot that starts at the twig’s end. As the rot progresses, internal vascular bundles show black to brown discoloration, eventually causing the infected twigs, branches, and stems to be completely engulfed by the rot (Fiorenza et al. 2023).
Additionally, Soil-borne diseases of avocados contribute to yield decrease and deterioration in fruit quality, limiting productivity and increasing costs. Under severe conditions, these diseases may lead to complete plant death, posing a serious threat to sustainable avocado production (Ramírez-Gil et al. 2017). Soil-borne fungal pathogens thrive and adapt well under conditions of excessive soil moisture, over-irrigation, and poor drainage, resulting in an altered structure and composition of the avocado rhizosphere microbial community (Solís-García et al. 2021). Infected trees produce a heavy crop of small fruits that decline and die either rapidly or gradually. Soil-borne fungal pathogens can spread through contaminated nursery stock, water in contact with infested soils, shoes, equipment, etc. (Ramírez-Gil et al. 2020). In tropical conditions, root rot is referred to as the avocado wilt complex (AWC), describing a multi-complex disease associated with different causal agents such as the oomycete P. cinnamomi, L. theobromae (Pat.) Griffon & Maubl. (Botryodiplodia theobromae), Fusarium spp., Rhizoctonia spp., Cylindrocarpon spp., Rosellinia spp., Pythium spp. (Vitale et al. 2012; Parkinson et al. 2017; Valencia et al. 2019). The implementation of proper and judicious management strategies is crucial for effective disease management.
To date, reported avocado diseases in Türkiye include fruit and leaf anthracnose caused by Colletotrichum spp., commonly associated with symptoms on avocado trees in the Mediterranean Region of Türkiye (Akgül et al. 2016; Uysal and Kurt 2020). Additionally, an outbreak of P. cinnamomi has led to severe decline and root rot of avocado trees in southern Türkiye (Akgül et al. 2016). These diseases present significant challenges to avocado cultivation in the region. However, it is crucial to conduct surveys on avocado diseases that cause substantial yield loss, monitoring and investigating outbreaks to effectively reduce such losses. Therefore, we conducted surveys in the most intense avocado production areas, observing more than two thousand trees in orchards. We documented the survey results, isolated fungal agents, and subsequently morphologically and molecularly identified them to understand the diseases causing severe symptoms. Our goal is to implement management strategies that reduce yield loss by addressing these identified diseases.
Material and methods
Survey and sampling
Avocado trees spanning different ages were meticulously chosen from both newly established and 40 years old aged orchards where were samples collected from 23 out of 28 total. Within each orchard, average of 1–7 selected trees were strategically spaced, 5–15 m apart (Supplementary Figure 1). Wood samples were diligently collected from infected orchards, stretching from Eastern Gazipasa (36.2686, 32.3143) to Western Kumluca (36.366858, 30.286996) provinces in Antalya, Türkiye. The orchards chosen for sampling were specifically identified based on previous reports to agricultural officers in those regions. From each tree 2–6 wood samples were extracted from the roots and trunks of affected trees, focusing on approximately 10–15 cm portions from feeder and secondary roots as well as discolored stems (Supplementary Figure 2). Concurrently, from each avocado tree, four rhizosphere soil samples, each weighing 5 g, were collected and subsequently homogenized into a single rhizosphere sample per tree, following the methodology outlined previously (Méndez-Bravo et al. 2018). This comprehensive sampling procedure was conducted during both the Autumn of 2020 and the Spring of 2021 seasons. To assess every determined orchard, the impact of the diseases, the percentage incidence and mortality of positively diagnosed trees were determined using a specified formula; Disease incidence \((\%) = No. of diseased plants/ Total No. of plants \times 100.\)
Isolation of fungi from collected samples
In the laboratory, the collected wooden and leaves samples underwent isolation using the twig, branch, and stem disinfection technique. Branch samples were thoroughly cleaned of surface dirt and debris using deionized water. Surface sterilization of the affected wooden and leaves samples ensued, involving the use of 80% ethyl alcohol (Merck, Germany) and 2% sodium hypochlorite (Ace, Türkiye), followed by subsequent rinsing in distilled sterilized water and air-drying on paper towels. The disinfected wooden samples were then transferred to a 2% potato dextrose agar (PDA) medium acidified with 10 ml of 25% lactic acid per liter. Incubation occurred at 28 ± 1 °C, with the grown isolates further incubated in the dark and identified (Supplementary Figure 3). Among overwhelming number of colonies formed after the incubation, those showed clear differences were selected for determining the fungus species. Pure fungal cultures were obtained by transferring hyphal tips or a piece of mycelia from the border of the actively growing colony onto fresh PDA medium.
Morphological evaluation of isolated fungi
To comprehensively assess the isolated fungi, a morphocultural evaluation was conducted. Besides observing colonial growth on PDA, the fungi were examined using a Leica DM750 microscope equipped with a K5Cmos/1 camera system (Leica, Germany). The fungal isolates were categorized into morphotypes based on colony characteristics, microscopic hyphae, conidia, and spore characters. All morphological and microscopic features were validated through comparison with previously described characteristics in different references (Robinson 2011; Hussain et al. 2012; Hafizi et al. 2014; Fiorenza et al. 2022, 2023; Hou et al. 2023; Pandey et al. 2020; Marquez et al. 2021; Abera et al. 2017).
Pathogenicity tests
In the experimental setup, 6-month-old branches were inoculated with isolated pathogens, including F. oxysporum, F. solani, P. cinnamomi, L. theobromae, and N. parvum. The pathogenicity tests were conducted on 3 sets of samples (three replications) using carefully cut 50 cm branches, and their edges were sealed with parafilm. Wounds were introduced 15 cm below each edge using a 5 mm diameter cork-borer, removing the bark to expose the cambium. To determine pathogenicity of isolated fungal species on Hass avocado branches, mycelial plugs from 1-week old isolated colony were then strategically placed into the wounds, with non-infested PDA plugs serving as controls. Wounds were covered with parafilm, and the inoculated branches were positioned in wet perlite for 25 days at 26 °C in a dark room to observe exclusively symptom development and measure internal vascular lesions without re-isolation since the plugs had already been purified colonies. The evaluation took place 25 days post-inoculation by longitudinally cutting the branches (Supplementary Figure 4b, c). For leaf inoculation (Supplementary Figure 4a), eight mycelial plugs in each replication were applied to the leaves of avocado (Hass), keeping the leaves alive by using wet cotton placement in the petiole. After 15 days, results were meticulously recorded. All records underwent analysis with ImageJ (University of Wisconsin, USA) to determine the areas of symptoms on leaves or branches by optimizing length with the plug (Supplementary Table 2–4). These experiments were repeated twice on each sampling conducted in two seasons, spring and autumn.
Molecular identification of isolated fungi
For molecular identification, total genomic DNA was extracted from the isolated fungi using the CTAB protocol. For DNA amplification, two primer sets were employed: ITS1-ITS2 and ITS5- ITS4 primers (Toju et al. 2012), targeting the complete ITS1 and ITS1 and ITS2 including 5.8S rDNA gene regions, respectively (Table 1). The PCR amplification utilized a total reaction mixture of 50 μl, comprising 2 μl of template DNA, 200 nmol L−1 of each primer, 1 μl DreamTaq Green PCR Mastermix (2 ×) (Thermo Fisher Scientific, USA), and 20 μl of nucleus-free water. To confirm amplification, the PCR products were run on a 1.5% agarose gel and visualized under UV light (Supplementary Figure 5). Cleaned amplicons of ITS2/ITS5 primers were then sent to Ficus Company (Ankara, Türkiye) for Sanger sequencing. Additionally, a specific and efficient method for detecting N. parvum, L. theobromae, F. oxysporum, and F. solani involved using species-specific primer pairs in PCR analyzes (Supplementary Table 1). The BioRad T100 Thermal Cycler (BioRad, USA) was utilized for amplification, with an initial denaturing step at 94 °C for 30 s, followed by 35 cycles of denaturing at 94 °C for 30 s, annealing at 57 °C for 30 s, extension for 1 min at 72 °C, and a final extension step of 5 min at 72 °C. The resulting PCR products were run on a 1.5% agarose gel and visualized under UV light using the Syngene (Syngene, USA) gel documentation system (Supplementary Figure 6).
Confirmation of F. oxysporum and F. solani was achieved using species-specific primers FnSc1/2 and TEF-Fs4-F/TEF-Fs4-R, respectively (Arif et al. 2012; Chang et al. 2022). N. parvum was confirmed as another pathogen with using Np304-F/Np304-R primers (Xu et al. 2016), with an intraspecies identification rate between different isolates ranging from 96.7 to 98.7%. Intriguingly, intraspecies identification for L. theobromae, verified with Lt347-F/Lt347-R primers (Xu et al. 2016),
Phylogenetic analyzes
The derived ITS data was employed to construct a phylogenetic tree and genetic matrix following pairwise alignment (70% similarity Cost Matrix, with free end gaps) in Geneious Prime. The phylogenetic tree was built using the Neighbor-Joining method, with Tamura-Nei serving as the Genetic Distance Model, and 100,000 bootstrap replicates for robustness. NR121224 (Penicillium citrunum), OM967428 (Corynospora torulosa) were utilized as the outgroups during tree construction to provide additional context. Simultaneously, a genetic identity matrix was generated and imported. A heatmap was constructed using the “seaborn” package in Python 3.12, offering a visual representation of the genetic relationships between the isolated fungi. Rooted tree is also available on Supplementary Figure 7 with rod and branch labels.
Statistical analyzes and data visualization
Data visualization involved utilizing the “matplot” library to generate box plots and bar graphs. Statistical analyzes, including ANOVA, were conducted using the “numpy” and “scipy” libraries in Python 3.9 by using PyCharm IDE.
Results
Avocado disease incidence and regional variations in antalya, Türkiye
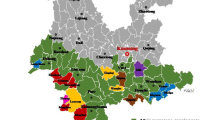
In total, 2537 avocado trees were surveyed across 11 different regions of Antalya, and the presence of dieback or leaf lesion symptoms was recorded from 2020 to 2021. Symptoms of dieback, branch canker, anthracnose, and soil-borne root rot were observed, affecting entire tree canopies, twigs, and branches. A total of 860 trees were identified as infected, resulting in an overall disease incidence of 33.89% within the surveyed orchards (Supplementary Table 5). Significant regional variations in disease incidence were observed, with higher rates in the easternmost parts of Antalya, specifically Gazipasa (GA-I, GA-II, and GA-III) and Alanya (AL-I, AL-II, and AL-III). Conversely, lower disease incidence rates were recorded in the central region of Manavgat (MA-I) and the western parts of Finike (FI-I) and Kumluca (KU-I, KU-II, and KU-III). Disease incidence rates were determined as 58.89% for GA, 32.54% for AL, 20.00% for MA and FI, and 14.75% for KU (Fig. 1). Furthermore, analyzing disease incidence based on the number of plants with dieback and canker symptoms revealed approximately 60% of trees in the 40-year-old orchard and 62% in the 1-year-old orchard were affected. Symptom severity was consistent in the 1-year-old orchard, with 60 to 65% of young trees exhibiting dieback of one to five twigs on the canopy tops, along with lesions longer than 10 cm.
Geographical distribution of collected samples from various districts in Antalya, highlighting disease incidence categorized by the number of infected and healthy trees. a Map representation with identified pathogens marked. b Stacked bar chart illustrating the number of infected trees alongside healthy ones. c Row charts displaying average disease incidence, with standard deviation bars; different colors and letters denote statistically distinct groups
Identification of isolated pathogens through colony and conidiospore characteristics
Following the isolation of pathogens from infected tissues, incubation on Potato Dextrose Agar (PDA) allowed for microscopy and observation of their growth. After 7–14 days of incubation, various colony morphologies, ranging from white fluffy to dark black mycelia growth, were observed. Subculturing and purification methods were employed to maintain these isolates. The colony characteristics of fungal species exhibited variations influenced by quantity, culture age, and cultural conditions. Based on the observed colony and conidiospore characteristics, the isolates were identified as F. solani, F. oxysporum, L. theobromae, N. rosae, C. gloeosporioides, N. parvum, M. phaseolina, and Phytophtora cinnamomi.
Fusarium species were detected on avocado trees are located in west
Samples collected from KU-I, KU-II, and KU-III were identified as F. solani and F. oxysporum based on their distinct morphological features. Colony characteristics of F. solani included white, cottony growth with floccose mycelium (Fig. 2). The colony margins were regular and smooth, exhibiting a slow growth rate. The underside of the colony displayed a pale to brown coloration. Microconidia were observed to be hyaline, oval, and some cylindrical with smooth edges, ranging from 5.02–8.52 × 2.91–5.50 μm (mean 6.88 × 3.79 μm). Macroconidia, slightly curved and broad with two to three septa, measured 13.05–34.18 × 2.10–5.50 μm (mean 18.85 × 3.36 μm). The number of macroconidia exceeded that of microconidia (Fig. 3), and these morphological characteristics were consistent with previous descriptions (Robinson 2011; Hafizi et al. 2014). F. oxysporum exhibited white to creamy aerial growth with smooth margins and occasionally slightly looped mycelia on PDA medium (Fig. 2). The lower side of the colony displayed a pale red to peach-violet coloration. Numerous ovoids to kidney-shaped microconidia without septa measured 11.2–19.9 × 4.5–8.4 μm (mean 15.4 × 6.1 μm). Macroconidia were thin-walled, falcate to almost straight, with both ends pointed and 2–3 septa, ranging from 22.1–43.9 × 5.1–12.5 μm (mean 28.4 × 7.5 μm). The number of macroconidia was lower than microconidia (Fig. 3), and their characteristics were consistent with previous descriptions.
Identification of species associated with canker and dieback
The colony of N. parvum exhibited a rough texture with irregular margins (Fig. 2). Initially, white dense filamentous aerial mycelia developed, eventually turning black over time. The black pycnidia were globose, either simple or aggregated. Conidia were bluntly round to subovoid, aseptate, and hyaline, with granular content (Fig. 3). They changed color from light brown to black, ranging from 19.77–15.25 × 4.10–7.5 μm (mean 17.01 × 5.70 μm). These morphological characteristics were consistent with previous descriptions N. rosae was identified by its colony characteristics, displaying aggregated white growth after 7 days of incubation at 26 °C. Additionally, it featured reduced conidiophores to short-cylindrical conidiogenous cells. Conidia were fusoid, straight or slightly curved, four-septate, smooth, slightly constricted at the septa, with the basal cell obconic and a truncate base (Fig. 3). They were thin-walled, hyaline, or pale brown, conforming to the characteristic shape reported previously (Fiorenza et al. 2022). L. theobromae was identified by its round and smooth colony. Initially, white aerial filamentous mycelia with a gray center were observed, gradually turning gray and then dark gray to black over time. The gray pycnidia were either simple or aggregated. Conidia were sub-ovoid to ellipsoid, initially aseptate, thick-walled, and hyaline, later forming a single medium septum and becoming dark brown (Fig. 3). The size ranged from 17.35–29.31 × 11.23–14.91 μm (mean 22.68 × 5.70 μm). M. phaseolina was identified based on colonies initially appearing white on PDA medium, later turning dark brown grayish (Fig. 2). Conidia were ellipsoid to obovoid, averaging 24.5 × 11.0 μm with a length–width ratio of 2.28. Immature conidia possessed apical mucoid appendages, and the morphological features were typical of M. phaseolina, consistent with previous descriptions.
Previously reported C. gloeosporioides and P. cinnamomi were detected
Following inoculation into Potato Dextrose Agar (PDA) and subsequent subculturing, isolated pathogens from MA-I, GA-I, GA-II, and GA-III were determined to be C. gloeosporioides. The identification was based on the distinctive growth pattern observed, covering the entire PDA plate after 8–10 days. The mycelial color of the isolate exhibited variations between whitish gray, whitish cream, and grayish pink on the upper side of the culture (Fig. 2). Similarly, the lower side of the cultures displayed shades of gray to creamish gray. The spores of C. gloeosporioides were cylindrical in shape, straight, with smooth round ends. The spore size ranged from 3.0–5.0 μm in width and 10.3–18.2 μm in length, aligning with the characteristics described previously. The cultural characteristics of P. cinnamomi were observed to include profuse tough aerial mycelium, at times forming a rosette pattern. The identification of P. cinnamomi was further supported by the presence of typical botryose hyphal swellings, which were round, aseptate, and often branched. Notably, the consideration of P. cinnamomi was based on the observation of branched hyphal swellings with a distinctive and peculiar shape (Fig. 3). However, it is noteworthy that P. cinnamomi was limited to AL-I, AL-II, and AL-III regions. It was not detected in samples collected from different distinct, highlighting a specific and localized distribution of this pathogen within the surveyed areas.
Molecular identification and genetic similarity of isolated pathogens
Following the determination of the Internal Transcribed Spacer (ITS) region of the isolated fungi, a phylogenetic tree was constructed to cluster the samples at the genus level. The identification between F. solani and F. oxysporum, based on ITS sequences, ranged from 79.8 to 82.5%, representing the highest identification score between two different species among the collected samples. Intraspecies identification within F. solani exhibited a high rate of 96.1–100%, marking the highest rate of intraspecies identification. Similarly, intraspecies identification for different isolates of F. oxysporum exceeded 95.8% (Fig. 4).
Phylogenetic analysis and genetic similarity matrix of isolated pathogens from avocado leaves, branches, and orchard soils. a Phylogenetic tree constructed using the Neighbor-Joining method with Tamura-Nei as the Genetic Distance Model (100,000 bootstrap). b Genetic similarity matrix based on ITS1 alignment of isolated pathogens
The intraspecific identification of L. theobromae reached 95.7–96.5%. The N. rosae, morphologically identified, demonstrated a high identification rate of 75.6% with F. oxysporum, a species belonging to the same dothideomyceta class as N. rosae. Conversely, the identification rate of C. gloeosporioides with Fusarium species was lower compared to N. rosae and N. parvum, ranging from 68.45 to 70.8% for F. solani and 68.7–72.1% for F. oxysporum.
The analysis also revealed the presence of other fungi in the collected samples from soils, such as Alternaria alternata (3 isolates), Corynespora torulosa (1 isolate), Entoleuca spp. (2 isolates), Botryotrichum murorum (1 isolate), and Fusarium fujikuroi (1 isolate). However, these fungi were not morphologically characterized or identified with species-specific primers in PCR. Subsequently, genetic similarity and a tree were constructed, considering only morphologically and molecularly confirmed isolated pathogens. Intraspecies genetic similarities were determined as follows: 96.3–98.9% for N. parvum (isolated 3 isolates), 94.4–94.6% for F. oxysporum (isolated 3 isolates), 93.9–100.0% for F. solani (isolated 7 isolates), and 97.7% for L. theobromae (isolated 2 isolates). This comprehensive molecular analysis provides a precise understanding of the genetic relationships among the isolated pathogens at both the species and intraspecies levels.
Pathogenic evaluation of isolated pathogens on avocado branches
P. cinnamomi, F. solani, and F. oxysporum were found to induce severe branch canker symptoms on avocado branches. Branches inoculated with L. theobromae and N. parvum exhibited less severe symptoms compared to those inoculated with P. cinnamomi, F. oxysporum, and F. solani. The branches inoculated with P. cinnamomi showed the highest lesion area, indicating a more aggressive impact on the avocado branches. Additionally, on average, lesion areas were higher in branches inoculated with P. cinnamomi, F. oxysporum, and F. solani, in comparison to branches inoculated with N. parvum and L. theobromae (Fig. 5).
Lesion areas on avocado (Hass) leaves and branches after pathogen inoculation. a Boxplots depicting lesion areas on branches recorded at the 25th day post-inoculation using ImageJ. b Average lesion values with standard deviation bars. c Lesion areas on leaves 15 days post-inoculation. d Bar graph displaying average lesion areas on leaves, with standard deviation bars. “n” denotes the number of inoculation series
Pathogenicity of inoculated pathogens on avocado leaves
Inoculation of N. rosae, N. parvum, and C. gloeosporioides isolates, onto avocado (Hass) leaves resulted in the induction of severe symptoms. The N. parvum-inoculated leaves exhibited extensive symptoms, covering the leaves with severe manifestations. Conversely, C. gloeosporioides demonstrated growth on inoculated leaves, presenting anthracnose-like symptoms. Notably, mycelial growth was observed on N. rosae-inoculated leaves. The most severe symptoms were observed on one of the inoculated disks of N. parvum, while N. rosae exhibited less severe growth. However, the statistical analysis revealed that the average of symptoms was comparable among leaves inoculated with different pathogens.
Discussion
Avocado stands as a vital fruit crop on the global scale, thriving in tropical and subtropical regions. Notably, it plays a significant role in international trade, with Europe being a major importer, particularly from the Mediterranean Region, including the southern areas of Türkiye. However, the susceptibility of avocado to various pathogens poses a substantial threat to both yield and quality in production. Considering this, a comprehensive survey of avocado orchards was conducted in Antalya, the largest avocado cultivation region in Türkiye, to assess the incidence of diseases. Covering 2537 avocado trees across 11 diverse regions from 2020 to 2022, the study identified a heightened risk of yield loss due to pathogens in the eastern part of Antalya. The presence of diverse pathogens, causing dieback, branch canker, anthracnose, and soil-borne root rot, was observed, significantly impacting tree canopies, twigs, and branches. The highest disease incidence, determined through dieback, anthracnose, or leaf spot evaluation, was noted in orchards situated in Gazipasa (58.89%) and Alanya (32.54%), located in the eastern part of Antalya, while Kumluca (16.75%), Finike (20.00%), and Manavgat (20.00%) in the western part exhibited comparatively lower disease incidence.
In the Mediterranean region, Colletotrichum species emerge as the primary culprits behind diseases afflicting avocado crops, particularly anthracnose. This fungal genus poses a significant threat to avocado fruits, causing a condition that not only results in substantial crop losses but also diminishes marketability. Notably, Colletotrichum karstii has been reported as a causal agent of fruit and leaf anthracnose disease in Türkiye (Uysal and Kurt 2020). However, in the current study, samples collected from the Gazipasa district in Antalya were identified as C. gloeosporioides based on morphological characteristics—cylindrical shape, straightness, and smooth round ends of spores—consistent with previous descriptions (Abera et al. 2017). This identification was further corroborated through ITS-based analysis, adding a molecular dimension to the verification process.
We identified P. cinnamomi based on morphological characteristics in samples collected from Alanya. Reports have documented an outbreak of P. cinnamomi, leading to a severe decline in avocado trees across southern Türkiye since the summer of 2017, particularly affecting 15–25 years old avocado trees in numerous commercial orchards situated in the Mediterranean coastal region of Türkiye (Kurbetli et al. 2020). Our study demonstrated that P. cinnamomi continues to inflict severe damage on avocado trees in the region. The root rot induced by P. cinnamomi stands out as a substantial threat to avocado crops, impacting the root systems of trees in this geographical area.
F. solani and F. oxysporum were identified in samples collected from the western part of Antalya within avocado orchards. These two species are widely associated with tropical fruit crops, known for their prevalence in the soil across tropical regions and their connection to vascular wilt diseases (Summerell et al. 2003). The identification of these species in our study was confirmed through both morphological analysis and molecular techniques, utilizing specific primers and ITS-based identification. While there have been no prior reports of F. solani and F. oxysporum in avocado trees from Türkiye, F. solani has been reported in the root and stem bark of avocado trees in Lebanon, situated in the Eastern Mediterranean (Abi Saad et al. 2022). Additionally, stem end rot caused by F. oxysporum and F. solani has been documented in Kenya.
The genetic diversity observed between F. solani and F. oxysporum was found to be narrow, while intraspecies diversity was lowest in F. solani, followed by F. oxysporum. Genetic diversity within the species F. solani can be influenced by various factors, including geographic location, host specificity, and reproductive behavior. Previous research has indicated that genetic diversity in F. solani can vary among different isolates and populations, potentially due to factors such as a low migration rate or gene-flow rate, as demonstrated in another study (De la Lastra et al. 2019). The observed low genetic diversity within intraspecies F. solani isolates may be attributed to specific environmental and biological factors, but further research is required to comprehensively understand the underlying reasons. Additionally, the genetic similarity matrix has consistently demonstrated a correlation with genetic differences between genus or family classifications.
L. theobromae and N. parvum have been previously identified as causal agents of dieback in avocado trees in Spain (Arjona-Girona et al. 2019). While L. theobromae has not been reported in avocado trees in Türkiye, it has been identified as a causal agent of dieback in almond (Prunus dulcis) trees in Türkiye during surveys in 2020 (Özer et al. 2022). L. theobromae has also been reported in avocado trees in Italy. Similarly, another dieback-causing pathogen, N. rosae, was reported in avocado plants in Italy alongside N. siciliana. The isolated N. rosae in our study exhibited similar morphological characteristics to those reported in a previous study (Fiorenza et al. 2022). M. phaseolina, another Botryosphaeriaceae species associated with canker and dieback disease in avocado, has been reported in avocado plants in Italy (Fiorenza et al. 2023). The pathogens isolated from Alanya and Finike were considered as M. phaseolina based on morphological evaluations of colony and spore characteristics.
The pathogenicity of the isolated pathogens was assessed by inoculating them onto avocado branches and leaves. The results revealed varying levels of symptom severity among different pathogens. P. cinnamomi, F. solani, and F. oxysporum were identified as causing severe branch canker symptoms, with P. cinnamomi resulting in the highest lesion area. In the case of avocado leaves, pathogens N. parvum, C. gloeosporioides, and N. rosae induced severe symptoms, including extensive leaf defoliation and anthracnose symptoms. The severity of these symptoms on leaves was not evaluated with statistically because these results were obtained with different pathogens and therefore could not compared among.
Briefly, we have identified F. oxysporum, F. solani, C. gloeosporioides, and N. parvum based on their morphology and confirmed their identification using both species-specific primers and the ITS-based method. The pathogenicity of these identified pathogens, along with N. rosae and L. theobromae, was assessed through inoculation on avocado (Hass) trees. Additionally, we tentatively identified other isolated pathogens such as M. phaseolina and P. cinnomomi based on their morphology, although confirmation with species-specific primers was not conducted in this study. Furthermore, we encountered different pathogens causing diseases in various plants, including A. alternata, B. murorum, and Entoleuca spp. These were not included in the ITS-based method due to a lack of comprehensive identification. Future studies can focus on a more detailed identification of these pathogens and further explore their pathogenicity in different plant species. The identified fungal species present in avocado orchards can be managed through a combination of cultural and chemical strategies aimed at reducing inoculum and preventing latent infections. However, long-term effective control remains challenging. Strategies such as the development of resistant avocado varieties and the exploration of biological management approaches are crucial for judiciously controlling branch canker, dieback, and anthracnose diseases in avocados. To achieve this, the development of an effective management program requires a multi-dimensional understanding of fungal etiology, epidemiology, evolution, environmental influences, and the intricacies of host–pathogen interactions. Implementing such a comprehensive approach is vital for reducing future crop losses and sustaining a healthy avocado industry.
References
Abera A, Adugna G, Lemessa F (2017) Prevalence and intensity of mango (Mangifera indica L.) anthracnose caused by colletotrichum species in South-western of Ethiopia. Ethiop J Appl Sci Technol 8(1):1–10
Abi Saad C, Masiello M, Habib W, Gerges E, Sanzani SM, Logrieco AF, Moretti A, Somma S (2022) Diversity of fusarium species isolated from symptomatic plants belonging to a wide range of agri-food and ornamental crops in Lebanon. J Fungi (basel) 8(9):897. https://doi.org/10.3390/jof8090897
Akgül DS, Awan QN, Güler PG, Önelge N (2016) First report of anthracnose and stem end rot diseases caused by colletotrichum gloeosporioides and neofusicoccum australe on avocado fruits in Turkey. Plant Dis 100(8):1792–1792. https://doi.org/10.1094/PDIS-03-16-0279-PDN
Arif M, Chawla S, Zaidi MW, Rayar JK, Variar M, Singh US (2012) Development of specific primers for genus Fusarium and F. solani using rDNA sub-unit and transcription elongation factor (TEF-1α) gene. Afr J Biotechnol 11(2):444–447. https://doi.org/10.5897/AJB10.489
Arjona-Girona I, Ruano-Rosa D, López-Herrera CJ (2019) Identification, pathogenicity and distribution of the causal agents of dieback in avocado orchards in Spain. Span J Agric Res 17(1):e1003. https://doi.org/10.5424/sjar/2019171-13561
Chang T-D, Huang L-N, Lin Y-J, Wu Z-B, Tsai S-H, Lin Y-H (2022) Rapid detection of fusarium oxysporum using insulated isothermal PCR and a rapid, simple DNA preparation protocol. Int J Mol Sci 23(21):13253. https://doi.org/10.3390/ijms232113253
De la Lastra E, Villarino M, Astacio JD, Larena I, De Cal A, Capote N (2019) Genetic diversity and vegetative compatibility of fusarium solani species complex of strawberry in Spain. Phytopathology 109(12):2142–2151. https://doi.org/10.1094/PHYTO-05-19-0173-R
Fiorenza A, Gusella G, Aiello D, Polizzi G, Voglmayr H (2022) Neopestalotiopsis siciliana sp. nov. and N. rosae causing stem lesion and dieback on avocado plants in Italy. J Fungi 8(6):562. https://doi.org/10.3390/jof8060562
Fiorenza A, Gusella G, Vecchio L, Aiello D, Polizzi G (2023) Diversity of Botryosphaeriaceae species associated with canker and dieback of avocado (Persea americana) in Italy. Phytopathol Mediterr 62(1):47–63. https://doi.org/10.36253/phyto-14057
Hafizi R, Salleh B, Latiffah Z (2014) Morphological and molecular characterization of Fusarium. solani and F. oxysporum associated with crown disease of oil palm. Braz J Microbiol 44(3):959–968
Hou X, Zheng M, Wang H, Hu X, Huang X, Jia Y, Zhou H (2023) Identification of Neofusicoccum parvum as the causative agent of leaf spot disease on Bletilla striata in China. Plant Dis 107(3):942. https://doi.org/10.1094/PDIS-03-22-0585-PDN
Hussain MZ, Rahman M, Islam MN, Latif M, Bashar M (2012) Morphological and molecular identification of Fusarium oxysporum Sch. Isolated from guava wilt in Bangladesh. Bangladesh J Bot 41(1):49–54. https://doi.org/10.3329/bjb.v41i1.11082
Kurbetli İ, Sülü G, Aydoğdu M, Woodward S, Bayram S (2020) Outbreak of Phytophthora cinnamomi causing severe decline of avocado trees in southern Turkey. J Phytopathol 168(9):533–541. https://doi.org/10.1111/jph.12931
Marquez N, Giachero ML, Declerck S, Ducasse DA (2021) Macrophomina phaseolina: general characteristics of pathogenicity and methods of control. Front Plant Sci. https://doi.org/10.3389/fpls.2021.634397
McDonald V, Eskalen A (2011) Botryosphaeriaceae species associated with avocado branch cankers in California. Plant Dis 95(11):1465–1473. https://doi.org/10.1094/PDIS-02-11-0136
Méndez-Bravo A, Cortazar-Murillo EM, Guevara-Avendaño E, Ceballos-Luna O, Rodríguez-Haas B, Kiel-Martínez AL, Hernández-Cristóbal O, Guerrero-Analco JA, Reverchon F (2018) Plant growth-promoting rhizobacteria associated with avocado display antagonistic activity against Phytophthora cinnamomi through volatile emissions. PLoS ONE 13(3):e0194665. https://doi.org/10.1371/journal.pone.0194665
Özer G, Türkölmez Ş, Derviş S (2022) First report of Lasiodiplodia theobromae causing dieback on almond (Prunus dulcis) in Turkey. J Plant Pathol 104(1):445–446. https://doi.org/10.1007/s42161-021-01018-6
Pandey AK, Burlakoti RR, Rathore A, Nair RM (2020) Morphological and molecular characterization of Macrophomina phaseolina isolated from three legume crops and evaluation of mungbean genotypes for resistance to dry root rot. Crop Prot 127:104962. https://doi.org/10.1016/j.cropro.2019.104962
Parkinson LE, Shivas RG, Dann EK (2017) Pathogenicity of nectriaceous fungi on avocado in Australia. Phytopathology 107(12):1479–1485. https://doi.org/10.1094/PHYTO-03-17-0084-R
Ramírez-Gil JG, Gilchrist Ramelli E, Morales Osorio JG (2017) Economic impact of the avocado (cv. Hass) wilt disease complex in Antioquia, Colombia, crops under different technological management levels. Crop Prot 101:103–115. https://doi.org/10.1016/j.cropro.2017.07.023
Ramírez-Gil JG, López JH, Henao-Rojas JC (2020) Causes of hass avocado fruit rejection in Preharvest, Harvest, and packinghouse: economic losses and associated variables. Agronomy 10(1):8. https://doi.org/10.3390/agronomy10010008
Robinson M (2011) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. Ref Rev 25(4):43–44
Solís-García IA, Ceballos-Luna O, Cortazar-Murillo EM, Desgarennes D, Garay-Serrano E, Patiño-Conde V, Guevara-Avendaño E, Méndez-Bravo A, Reverchon F (2021) Phytophthora root rot modifies the composition of the avocado rhizosphere microbiome and increases the abundance of opportunistic fungal pathogens. Front Microbiol. https://doi.org/10.3389/fmicb.2020.574110
Summerell BA, Salleh B, Leslie JF (2003) A utilitarian approach to fusarium identification. Plant Dis 87(2):117–128. https://doi.org/10.1094/PDIS.2003.87.2.117
Toju H, Tanabe AS, Yamamoto S, Sato H (2012) High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 7(7):e40863. https://doi.org/10.1371/journal.pone.0040863
Uysal A, Kurt Ş (2020) First report of fruit and leaf anthracnose caused by Colletotrichum karstii on avocado in Turkey. Crop Prot 133:105145. https://doi.org/10.1016/j.cropro.2020.105145
Valencia AL, Gil PM, Latorre BA, Rosales IM (2019) Characterization and pathogenicity of Botryosphaeriaceae Species obtained from avocado trees with branch canker and dieback and from avocado fruit with stem end rot in chile. Plant Dis 103(5):996–1005. https://doi.org/10.1094/PDIS-07-18-1131-RE
Vitale A, Aiello D, Guarnaccia V, Perrone G, Stea G, Polizzi G (2012) First report of root rot caused by Ilyonectria (=Neonectria) macrodidyma on avocado (Persea americana) in Italy. J Phytopathol 160(3):156–159. https://doi.org/10.1111/j.1439-0434.2011.01869.x
Wanjiku EK, Waceke JW, Wanjala BW, Mbaka JN (2020) Identification and pathogenicity of fungal pathogens associated with stem end rots of avocado fruits in Kenya. Int J Microbiol 2020:e4063697. https://doi.org/10.1155/2020/4063697
Xu C, Zhang H, Chi F, Ji Z, Dong Q, Cao K, Zhou Z (2016) Species-specific PCR-based assays for identification and detection of Botryosphaeriaceae species causing stem blight on blueberry in China. J Integr Agric 15(3):573–579. https://doi.org/10.1016/S2095-3119(15)61177-7
Acknowledgements
This study was supported by The Scientific Research Projects Coordination Unit of Akdeniz University (Project Number: FYL-2021-5520). The authors would like to thank Mustafa KAYA for excellent guidance in orchards.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Here, all authors have no conflict of interest to declare.
Ethical approval
Here, we have confirmed that the manuscript complies to the Ethical Rules applicable for this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çalış, Ö., Çelik, S., Fidan, H. et al. Emerging pathogens and disease dynamics threatening avocado production in southern Türkiye. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00954-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00954-6