Abstract
Here we investigated the effect of the insect pest whitefly (Bemisia tabaci), cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) on cassava root yield and quality in two cropping seasons 2014–2015 and 2015–2016 on ten cassava varieties in Tanzania. ANOVA (sum of squares or SS) revealed that the time of planting (42.7%) and cassava variety (29.5%) had the largest effect on whitefly population. Not surprisingly, cassava varieties also had the highest effect (SS 39.8 to 70.4%) on both diseases and yield. An increase in whitefly population led to higher disease incidences and severity in 2015–2016 compared to 2014–2015. Some CBSD-resistant and tolerant cassava varieties like Namikonga and Kiroba, respectively, harboured high whitefly populations. The CMD, CBSD and whitefly-susceptible variety, Mreteta, showed highest yield losses of up to 60%, while the resistant variety NDL 2005/1471 had approximately 1% loss. Deployment of varieties resistant to both diseases and whitefly is thus necessary to safeguard cassava production and food security of vulnerable communities in the affected African countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassava (Manihot esculenta Crantz) is an important staple food in sub-Saharan Africa (SSA), ranked second after maize in Eastern and Southern African countries (FAOSTAT 2020). With approximately 200 million metric tonnes of annual root production, cassava is a major source of carbohydrates in the diet of about 450 million people in SSA. It is also grown as a famine reserve crop owing to its tolerance to harsh environmental conditions (Jarvis et al. 2012). The crop has enormous potential as an essential economic driver within the agriculture sector in SSA countries for exploitation in industries to produce high-quality cassava flour, starch, beverages and animal feed (Luar et al. 2018). Despite its importance, the average cassava yield in Africa is, however, about 9.0 t/ha (FAOSTAT 2020), which is well below the yield potential of 50–90 t/ha achieved under optimal conditions (Ntawuruhunga et al. 2006; El-Sharkawy 2004; Obiero 2004). However, average yields in the Eastern African countries of Uganda, Tanzania and Kenya are very low at 3.3, 7.3, and 14.5 t/ha, respectively (FAOSTAT 2020).
One of the factors for the low yield is severe damage caused by insect pests and diseases that hinder cassava production (Ekeleme et al. 2017; Ezui et al. 2016). Two of the most critical current biotic constraints in Eastern and Southern Africa are viral diseases: cassava mosaic disease (CMD) caused by cassava mosaic begomoviruses (CMBs) (family Geminiviridae) and cassava brown streak disease (CBSD) caused by cassava brown streak ipomoviruses (CBSIs) (family Potyviridae) (Legg et al. 2011, 2015; Mohammed et al. 2012). CMD symptoms typically include irregular yellow or yellow-green chlorotic mosaic pattern on leaves, leaf distortion, stunted plant growth and reduced or complete root yields, but not rotting of roots (Storey and Nichols 1938; Thresh and Cooter 2005; Tembo et al. 2017). The most damaging effect of CBSD is root necrosis, causing yield losses of up to 75% as the root is unmarketable or inedible in the most susceptible varieties (Maruthi et al. 2020). These diseases together can severely reduce cassava productivity in sub-Saharan Africa, causing annual losses of up to US$3 billion to resource poor farmers (Thresh et al. 1997; Hillocks and Maruthi 2015).
The whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is a serious plant pest and is the sole vector of CMBs and CBSIs in cassava (Legg 2010; Maruthi et al. 2005, 2017). Whitefly feeding on cassava can also damage plants causing chlorotic mottling, twisting or curling, particularly on upper leaves (Bellotti and Arias 2001). Large populations that develop early in the crop's life reduce plant vigour and tuber sizes and cause stunted growth leading to more than 50% loss in yield (Legg et al. 2004). A large whitefly population can also produce honeydew, which leads to the production of black sooty mould on lower leaves, reducing the photosynthetic ability of the plant, further contributing to yield losses (Legg et al. 2004; Omongo et al. 2004). However, the most significant economic threat is spread of CMD and CBSD.
Disease and whitefly prevalence surveys have been conducted in the past to assess the epidemiology of both CMD and CBSD (Muhindo et al. 2020; Tairo et al. 2019; Harimalala et al. 2015; Legg et al. 2011). These findings had shown high whitefly abundance with high CMD and CBSD severity and incidences. However, few studies have investigated the cumulative effects of damage by whiteflies and viral diseases on cassava. To investigate this, we evaluated the spread of whiteflies, CMD, CBSD and their impact on cassava yield and quality in two cropping seasons in Tanzania.
Materials and methods
Cassava germplasm and screening location
Ten popularly grown cassava varieties and advanced breeding lines were selected for this study (Table 1). The field trials were established in 2014–2015 and 2015–2016 cropping seasons in the CMD and CBSD hot spot research fields of the Tanzania Agricultural Research Institute (TARI)—Naliendele in the Mtwara region of southern Tanzania. TARI-Naliendele lies on the coastal belt of the Indian Ocean at 10° 22′ 20"S, 40° 10′ 34"E and 111 m above mean sea level. The area receives the main rainfall from December to May, with second rains of scattered showers in August–October (Dondeyne et al. 2003). The sandy soils of the Mtwara region are considered poor for most crops. They comprise deep, well-drained, weak-structured dark reddish-brown loamy sand topsoil over reddish brown moderately structured sandy loam to sandy clay loam subsoil (Dondeyne et al. 2003).
Experimental design
The field trials were laid out using the Randomised Complete Block Design (RCBD) with three replications and a plot size of 4 m × 10 m. The first trial was planted on 12 January 2014 and harvested on 20 January 2015, while the second was planted on 16 March 2015 and harvested on 19 March 2016 at the Naliendele site. Both trials consisted of 10 cassava varieties and advanced breeding lines, namely NDL2003/031, NDL2003/111, NDL2005/1471, KBH96/1056, KBH 2002/494, KBH2002/482, Kiroba and Mreteta. The control checks included Namikonga (susceptible and resistant to CMD and CBSD, respectively) and Albert (resistant to CMD and susceptible to CBSD) (Table 1). Mature cassava cuttings of about 25 cm long and having 4–5 nodes with viable buds were collected for each variety from TARI—Makutupora in Tanzania (a disease-free site used for seed multiplication). To increase disease inoculum, CMD-susceptible variety Limbanga and CBSD-susceptible variety Albert were planted around the experimental plots as spreader rows (Kundy et al. 2014). The trial was rain-fed and kept weed-free by monthly weeding, and no fertiliser was applied.
Epidemiology and effect of cassava viral diseases and insect pests on yield
Twenty plants per plot were selected from the two inner rows for data collection from 1 to 12 months after planting. The data collected included whitefly adult count on top five leaves, CMD and CBSD foliar severities, CBSD root symptoms (root necrosis), root weight (t/ha), marketable roots (t/ha) and dry matter content from 20 plants/plot/variety. The foliar severity for CMD was scored on a 1–5 scale where: 1 = no visible symptoms; 2 = mild distortion only at the base of leaflets with the rest of leaflets appearing green and healthy/mild chlorotic pattern over the entire leaflets; 3 = conspicuous mosaic pattern throughout the leaf, narrowing and distortion of lower 1/3 of leaflets; 4 = severe mosaic, distortion of two-thirds of leaflets and general reduction in leaf size; and 5 = severe mosaic, distortion of ¾ of leaflets, twisted and malformed leaves (Hahn et al. 1980). Foliar severity for CBSD was scored on a 1–5 scale where: 1 = no visible symptoms; 2 = mild foliar mosaic on some leaves and no stem lesions; 3 = foliar mosaic with mild stem lesions and no dieback; 4 = foliar mosaic and pronounced stem lesions and no die back; and 5 = defoliation with pronounced stem lesions and dieback (Hillocks et al. 1996).
At about 12 MAP, plants were harvested, and roots were examined for root necrosis. Roots from each plant were chopped longitudinally and transversely to identify the presence of necrotic patches on the starch-bearing tissues. Scoring for root necrosis severity was also done based on a 1–5 scale where: 1 = no clear symptoms; 2 = < 5% of root necrotic; 3 = 5 –25% of root necrotic; 4 = 25–50% root necrotic and mild root constriction; and 5 = > 50% of root necrotic (Masinde et al. 2016; Hillocks and Maruthi 2015; Gondwe et al. 2002). All roots with a necrosis score of ≤ 2 were considered marketable as only tiny spots of root necrosis were observable at this score (Masinde et al. 2016). Severe root necrosis affects root quality, reducing the quantity of marketable roots. Marketable roots per variety were determined by deducting the unmarketable roots with root necrosis score > 2 from the total roots.
Root weight in tonnes per hectare (t/ha/) was estimated according to Masinde et al. (2017).
Further, the specific gravity method collected data on root dry matter content (Kawano 1987).
Data analysis
Data analysis was performed using R packages multcomp, agricolae and emmeans. A 3-way ANOVA was performed on the effect of time of planting (months after planting, MAP), variety and season for whitefly infestation, CMD and CBSD foliar severities. A 2-way ANOVA was performed on the effects of variety and season for root necrosis and yield parameters including root weight, marketable roots and dry matter content. Treatment means were separated using the LSD test at a 95% confidence level. Graphs were plotted for whitefly abundance and yield traits, while means were calculated for the ratings on colour, smell and taste of cassava products used in the organoleptic test.
Results
Evaluation of different traits
The time of planting (MAP) had the largest effect on whitefly abundance (Table 2) (42.7% of the total SS), followed by variety (29.5%) and interaction effect between MAP and season (13.7%). Additionally, the mean squares were highly significant (P ≤ 0.001) for the factors and their interactions. The cassava varieties contributed to the maximum differences (39.8 to 70.4% of SS) observed for CMD, CBSD, root necrosis, root weight and marketable roots (Table 2 and 3). Seasonal effect accounted for 12.4 to 43.1% SS, while variety by season interaction accounted for 5.1 to 16.9% differences. The mean squares for the factors and most of their interactions were significant (P ≤ 0.05) (Table 2 and 3). Finally, the season (time of harvesting) had the largest effect (83.7% SS) on dry matter content (Table 3) with minor effect by the varieties (9.7%). Generally, a large SS for a factor indicates that it contributes to most variations observed.
Whitefly abundance and morphological characterisation of varieties
Cropping season 2015—2016 had a slightly higher mean number of whiteflies ranging from 0.5 to 28.5 compared to 2014–2015 (0.5–16.4) (Figs. 1 and 2). The mean whitefly population increased from 1 MAP and peaked at approximately 6 MAP (16.4) and 4 MAP (28.5) in 2014–2015 and 2015–2016, respectively. The population declined sharply after with the lowest count of 0.5 by 12 MAP in both seasons. Whitefly count varied significantly (P ≤ 0.05) among the varieties. Vars. Namikonga and Mreteta had the highest count ranging from 1.1 to 50.7, while Albert, KBH 2002/482, KBH 2002/494, KBH 96/1056 and Kiroba had moderate counts (0.0–31.3) across the seasons. Vars. NDL 2003/031, NDL 2003/111 and NDL 2005/1471 had the least count ranging from 0.0 to 13.9 across the seasons.
The leaf colour, the shape of the central leaflet, the orientation of petioles and the hairiness/smoothness of leaves formed the basis for the morphological characterisation of cassava varieties for whiteflies' preference and colonisation. Accordingly, varieties with the highest whiteflies count, including Namikonga and Mreteta, had light green foliage, unlike the rest, which had dark green foliage (Fig. 3). Varieties with least whiteflies count, including NDL 2003/031, NDL 2003/111 and NDL 2005/1471, had lanceolate-shaped central leaflets, unlike the others whose leaves were elliptical–lanceolate. Petioles for Albert and KBH 2002/494 were inclined downwards, Kiroba and KBH 96/1056 inclined horizontally, while KBH 2002/482, Namikonga, Mreteta, NDL 2003/031, NDL 2003/111, and NDL 2005/1471 had petioles facing upwards. NDL 2003/031, NDL 2003/111 and NDL 2005/147, the least infested varieties, possessed slightly hairy leaves while the rest had smooth leaves.
CMD foliar incidence and severity
2015–2016 had the highest mean CMD foliar severities and whitefly count (Table 4). In 2014–2015, CMD foliar symptoms were first observed at 2 MAP, while in 2015–2016 were observed very early at 1 MAP. In most varieties, CMD foliar severity increased throughout the two growing seasons and peaked between 6 and 8 MAP before dropping up to 12 MAP. Var. Mreteta had the highest CMD foliar severity ranging from 1.0 to 3.0, while KBH 96/1056 had the least ranging from 1.0 to 1.2 across the seasons.
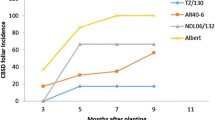
CBSD foliar incidence, severity and root necrosis
CBSD foliar symptoms were first observed at 3 and 2 MAP in 2014–2015 and 2015–2016, respectively. 2015–2016 also had the highest mean CBSD foliar severities and whitefly count (Table 5). In 2014–2015, the mean CBSD foliar severity increased throughout the growing season until 9 MAP, then dropped from 10 to 12 MAP. In contrast, the mean CBSD foliar severity increased to 7 MAP in 2015–2016, then dropped from 8 to 12 MAP. Varieties with significantly (P ≥ 0.05) high CBSD foliar severity also had high root necrosis and vice-versa. Accordingly, var. Albert had the highest CBSD foliar severity ranging from 1.0 to 3.1 and root necrosis severity ranging from 2.4 to 2.7 across the seasons while Namikonga had the least CBSD foliar severity (1.0–1.3) and root necrosis (1.0–1.1).
Cassava yield traits
2014–2015 had significantly (P ≥ 0.05) higher root weight, marketable roots and dry matter content, and it also received higher rainfall and had the least disease and whitefly incidences (Figs. 4 and 5). Varieties with significant differences between the two seasons in disease and whitefly incidences also had significant differences in root weight. For example, var. Mreteta had a low CMD incidence of (0.0–14.7%) with a root weight of 21.5 t/ha in 2014–2015, while it had a higher incidence of (44.1–75.6%) with a low root weight of 12.8 t/ha in 2015–2016 (Table 4, Fig. 4). On the other hand, NDL 2005/1471 had low CMD severities in both seasons and hence the minimal difference in root weight of 25.4 t/ha and 23.4 t/ha in 2014–2015 and 2015–2016, respectively (Table 4, Fig. 4).
Varieties with least root necrosis severity and high root weight also had higher quantities of marketable roots. Accordingly, NDL 2003/1471 had the highest quantity of marketable roots of 25.3 t/ha in 2014–2015 and 23.0 t/ha in 2015–2016, while Albert had the least: 11.0 t/ha in 2014–2015 and 3.9 t/ha in 2015–2016 (Fig. 5). Although Namikonga had low root necrosis severity in both seasons, it had a low quantity of marketable roots, 14.7 t/ha in 2014–2015 and 10.7 t/ha in 2015–2016 due to its low root yield.
Higher levels of dry matter content (22.9–28.4%) were recorded in 2014–2015 compared to 2015–2016 (18.8–20.1%). Season 2015–2016 had higher whitefly and disease incidences, which may have contributed to the low dry matter content recorded (Table 4, 5). Further, a higher quantity of rainfall between January and March in season 2015–2016 may have contributed to lower dry matter content.
Discussion
The cassava varieties, season and their interactions were significant (P ≥ 0.05) in all the parameters evaluated in this study. The MAP had the largest SS, signifying that it contributed to the major variations observed for the whitefly population. Cassava leaves began emerging 7 and 10 days after planting, and adult whitefly were detected at 1 MAP. The whitefly population peaked at 6 and 4 MAP in 2014–2015 and 2015–2016, respectively, before dropping sharply by 12 MAP. It has been known that suitability of cassava for whitefly feeding decreases as it matures due to the hardiness of leaves.
Whitefly population usually starts building up early when leaves are young and just formed, and peaks between 5 and 7 MAP when the foliage is well formed and succulent, after which it drops drastically as the plant grows older, becomes woodier and sheds leaves (Macfadyen et al. 2018; Sseruwagi et al. 2005). The whitefly population was higher in season 2015–2016 than 2014–2015. This may be due to delayed planting in March 2015, which has allowed build of high number of whiteflies on neighbouring crops and weeds and moving on to the young succulent cassava plants in our trials (Mohamed 2012). The temperatures are generally high, above 30 °C, at the study site Naliendele from February to May (Kimata et al. 2021), which is highly favourable for whitefly development and will have also contributed to the early peaks in populations seen in this study (Shirima et al. 2019).
The varietal effect had the second largest SS, contributing to major variations observed for the whitefly population. Morphological characterisation of cassava varieties regarding whitefly preference and colonisation confirmed the characteristic traits of resistant and susceptible varieties. NDL 2003/031, NDL 2003/111 and NDL 2005/1471 were the least infested and were categorised as resistant, while Namikonga and Mreteta were susceptible. The resistant varieties had dark green foliage, slightly hairy leaves and lanceolate-shaped central leaflets, unlike the susceptible ones with light green foliage, smooth leaves and elliptical–lanceolate-shaped central leaflets. Similar results were observed when whiteflies preferentially fed and oviposited more on varieties with light green and smooth foliage (Gwandu et al. 2019). Leaf hairs interfere with whitefly landing and feeding on cassava; therefore, it is a likely trait of resistance (Gwandu et al. 2019; Byrne and Bellows 1991). Additionally, dark green leaf varieties are known to have higher phenolic content, which act as feeding deterrents and ultimately providing resistance against insects such as whiteflies (Shibuya et al. 2010; Mwila et al. 2017; Chu et al. 2017). Selection of cassava for whitefly resistance can therefore include varieties with darker green foliage as a trait in cassava breeding.
The cassava variety contributed to the largest SS for CMD, CBSD and root necrosis. Vars. Namikonga, Mreteta and Kiroba had the highest CMD and/or CBSD severity and a high whitefly population. Vars. Namikonga and Kiroba expressed field resistance and tolerance, respectively, to CBSD, and breeders commonly use them as the best sources of CBSD resistance/tolerance in conventional breeding programs. This should be reconsidered to prevent the development of whitefly-susceptible varieties which was partially responsible for the development of high whitefly populations on cassava in eastern Africa (Macfadyen et al. 2018). The incidences of CBSD decreased after 9 MAP in both years, which is the result of severe dropping of older leaves with CBSD symptoms due to prolonged dry periods (less than 10 mm rainfall per month) experienced from May to October at the study site Naliendele (Kimata et al. 2021).
Season 2014–2015 had lower root weight, marketable roots and dry matter content, probably due to a higher disease incidence. Additionally, low dry matter content was recorded in all varieties due to harvesting during the rainy season in March 2016. Low root necrosis severity coupled with high root weight results in high quantity of marketable roots and vice versa (Maruthi et al. 2020; Masinde et al. 2017). Var. NDL 2005/1471 had the highest quantity of marketable roots, unlike Namikonga, which, although it expressed minimal root necrosis, still had low quantity of marketable roots due to an initial low root weight. Namikonga is a low-yielding variety besides developing CMD symptoms, reducing yield (Masumba et al. 2017; Kaweesi et al. 2014). The high whitefly population in Namikonga could also be contributing to its low yields since the pest feeds on the phloem.
In 2014–2015 season, which had a low disease and whitefly incidence, var. Mreteta had root weight (21.5 t/ha) and marketable roots (13.2 t/ha), translating to 37% yield loss. In 2015–2016, higher disease and whitefly incidence led to lower root weight of 12.5 t/ha and marketable root (5.0 t/ha), translating to 60% loss. In contrast, the resistant var. NDL 2005/1471 had root weight (25.8 t/ha) in 2014–2015 with marketable roots (25.8 t/ha), translating to 1.2% yield loss. Similarly, in 2015–2016, NDL 2005/1471 had approximately 0.4% yield loss. It is therefore important to grow cassava varieties that are resistant to both whiteflies and CMD and CBSD, to achieve the full yield potential of cassava and thus increase high cassava production by the farmers.
Conclusions
Whitefly can spread viruses causing both CMD and CBSD, and if they all occur concurrently in a cropping season, they can result in significant losses of cassava root yield and quality. Seasonable variations were observed as 2015–2016 had higher whitefly population than 2014–2015, resulting in higher CMD and CMD foliar severities and consequently lower root yield and quality. Varietal variations were also observed as whitefly had higher preference for Namikonga, Kiroba and Mreteta, which also developed severe symptoms of either CMD, CBSD or both diseases. Planting a variety like Mreteta, susceptible to whitefly, CMD and CBSD, can make a farmer incur significant losses up to 60%, while the resistant var. NDL 2005/1471 suffered an approximate loss of only 1%. This study demonstrated that some varieties popularly used as breeding sources for CMD and CBSD resistance are susceptible to whitefly (e.g. Namikonga). Considerations should therefore be given to deploying high-yielding varieties resistant to both whiteflies, and CMD and CBSD to increase cassava productivity and food security in African countries.
References
Bellotti AC, Arias B (2001) Host plant resistance to whiteflies with emphasis on cassava as a case study. Crop Prot 20:813–823. https://doi.org/10.1016/S0261-2194(01)00113-2
Byrne DN, Bellows TS (1991) Whitefly biology. Annu Rev Entomol 36:431–457. https://doi.org/10.1016/B978-0-12-802441-6.00004-8
Chu B, Zhang S, Wang L, Zhu X, Luo J, Wang C, Lü L, Cui J (2017) Genetic regulation of defence responses in cotton to insect herbivores. J Asia Pac Entomol 20:341–351. https://doi.org/10.1093/aobpla/plx048
Dondeyne S, Ngatunga EL, Cools N, Mugogo S, Deckers J (2003) Landscapes an soils of south-eastern Tanzania: Their sustainability for cashew. In: Topper CP, Kasuga LJ (eds) Knowledge transfer for sustainable tree crop development-a case history of the Tanzanian integrated cashew management programme. BioHydrids Agrisystems Limited, UK, pp 229–239
Ekeleme F, Hauser S, Atser G, Dixon A, Weller S, Olorunmaiye P, Usman H, Olojede A, Chikoye D (2017) Weed management in cassava in Africa: challenges and opportunities. Outlooks Pest Manag 27:208–212. https://doi.org/10.1564/v27_oct_04
El-Sharkawy MA (2004) Cassava biology and physiology. Plant Mol Biol 56:481–501. https://doi.org/10.1007/s11103-005-2270-7.s
Ezui KS, Franke AC, Mando A, Ahiabor BDK, Tetteh FM, Sogbedji J, Janssen BH, Giller KE (2016) Fertilizer requirements for balanced nutrition of cassava across eight locations in West Africa. Field Crops Res 185:69–78. https://doi.org/10.1016/j.fcr.2015.10.005
Food and agriculture organization corporate statistical database (FAOSTAT), 2020. Food and agriculture data accessed 12.06.22. http://www.fao.org/faostat/en/#data/
Gondwe FMT, Mahungu NM, Hillocks RJ, Raya MD, Moyo CC, Soko MM, Chipungu FP, Benesi IRM (2002) Economic losses experienced by small-scale farmers in Malawi due to cassava brown streak virus disease. In: Legg JP, Hillocks RJ (Eds.) Cassava brown streak virus disease: Past, present and future proceedings of an international workshop Mombasa, Kenya. Natural Resources International Limited, Aylesford. pp. 28–36
Gwandu C, Ochwo-Ssemakula M, Sseruwagi P (2019) Whitefly resistance in African cassava genotypes. Afr Crop Sci J 27:213–228. https://doi.org/10.4314/acsj.v27i2.7
Hahn SK, Terry ER, Leuschner K (1980) Breeding cassava for resistance to cassava mosaic disease. Euphytica 29:673–683. https://doi.org/10.1007/BF00023215
Harimalala M, Chiroleu F, Giraud-Carrier C, Hoareau M, Zinga I, Randriamampianina JA, Velombola S, Ranomenjanahary S, Andrianjaka A, Reynaud B, Lefeuvre P, Lett JM (2015) Molecular epidemiology of cassava mosaic disease in Madagascar. Plant Pathol 64:501–507. https://doi.org/10.1111/ppa.12277
Hillocks RJ, Maruthi MN (2015) Post-harvest impact of cassava brown streak disease in four countries in Eastern Africa. Food Chain 5:116–122. https://doi.org/10.3362/2046-1887.2015.008
Hillocks RJ, Raya M, Thresh JM (1996) The association between root necrosis and above-ground symptoms of brown streak virus infection of cassava in Southern Tanzania. Int J Pest Manag 42:285–289. https://doi.org/10.1080/09670879609372008
Jarvis A, Ramirez-Villegas J, Herrera Campo BV, Navarro-Racines C (2012) Is cassava the answer to African climate change adaptation? Trop Plant Biol 5:9–29
Kawano K (1987) Inherent and environmental factors related to cassava varietal selection. In: Hershey E (ed) Cassava breeding: a multidisciplinary review. CIAT, Cali, pp 207–226
Kaweesi T, Kawuki R, Kyaligonza V, Baguma Y, Tusiime G, Ferguson ME (2014) Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virol J 11:216. https://doi.org/10.1186/s12985-014-0216-x
Kimata B, Masinde E, Masisila F, Menya R, Matondo D, Maruthi MN, Mkamilo G (2021) Adaptability and phenotypic stability of resistance to two viral diseases and yield traits in cassava. Am J Plant Sci 12:679–705. https://doi.org/10.4236/ajps.2021.124046
Kundy AC, Mkamilo GS, Misangu RN (2014) Assessment and selection of superior genotypes among elite cassava genotypes by farmers and scientists in Southern Tanzania. J Nat Sci Res 4:17
Legg JP (2010) Epidemiology of a whitefly transmitted cassava mosaic geminivirus pandemic in Africa. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Berlin, pp 233–257
Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, Okao-Okuja G, Bouwmeester H, Bigirimana S, Tata-Hangy W, Gashaka G, Mkamilo G, Alicai T, Lava Kumar P (2011) Comparing the regional epidemiology of the cassava mosaic and cassava brown streak pandemics in Africa. Virus Res 159:161–170. https://doi.org/10.1016/j.virusres.2011.04.018
Legg JP, Lava Kumar P, Makeshkumar T, Ferguson M, Kanju E, Ntawuruhunga P, Tripathi L, Cuellar W (2015) Cassava virus diseases: biology, epidemiology and management. Adv Virus Res 91:85–142. https://doi.org/10.1016/bs.aivir.2014.10.001
Legg JP, Sseruwagi P, Brown J (2004) Bemisia whiteflies cause physical damage and yield loss to cassava in Africa. In: The 6th international scientific meeting of the cassava biotechnology network. CIAT, Cali, Colombia, p. 78
Luar L, Pampolino M, Ocampo A, Cordora DF, Oberthur T (2018) Cassava response to fertilizer application. Better Crops 102:11–13. https://doi.org/10.24047/BC102211
Macfadyen S, Paull C, Boykin LM, De Barro P, Maruthi MN, Otim M, Kalyebi A, Vassão DG, Sseruwagi P, Tay WT, Delatte H, Seguni Z, Colvin J, Omongo CA (2018) Cassava whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in East African farming landscapes: A review of the factors determining abundance. Bul Entomol Res 5:1–18. https://doi.org/10.1017/S0007485318000032
Maruthi MN, Hillocks RJ, Mtunda K, Raya MD, Muhanna M, Kiozia H, Rekha AR, Colvin J, Threshh JM (2005) Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J Phytopathol 153:307–312. https://doi.org/10.1111/j.1439-0434.2005.00974.x
Maruthi MN, Jeremiah SC, Mohammed IU, Legg JP (2017) The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J Phytopathol 165:707–717. https://doi.org/10.1111/jph.12609
Maruthi MN, Kimata B, Masinde EA, Mkamilo G (2020) Effect of time of harvesting and disease resistance in reducing cassava (Manihot esculenta Crantz) yield losses by two viral diseases. Mod Concepts Dev Agron 6:601–616. https://doi.org/10.31031/MCDA.2020.06.000628
Masinde EA, Ogendo J, Mkamilo G, Maruthi MN, Hillocks R, Mulwa RMS, Arama PF (2016) Occurrence and estimated losses caused by cassava viruses in Migori County. Kenya Afr J Agric Res 11:2064–2074. https://doi.org/10.5897/AJAR2016.10786
Masinde EA, Mkamilo G, Ogendo J, Hillocks R, Mulwa RMS, Kimata B, Maruthi MN (2017) Genotype by environment interactions in identifying cassava (Manihot esculenta Crantz) resistant to cassava brown streak disease. Field Crops Res 215:39–48. https://doi.org/10.1016/j.fcr.2017.10.001
Masumba EA, Kapinga F, Mkamilo G, Salum K, Kulembeka H, Rounsley S, Bredeson JV, Lyons JB, Rokhsar S, Kanju E, Katari MS, Myburg AA, van der Merwe NA, Ferguson ME (2017) QTL associated with resistance to cassava brown streak and cassava mosaic diseases in a bi-parental cross of two Tanzanian farmer varieties, Namikonga and Albert. Theor Appl Genet 130:2069–2090. https://doi.org/10.1007/s00122-017-2943-z
Mohamed MA (2012) Impact of planting dates, spaces and varieties on infestation of cucumber plants with whitefly, Bemisia tabaci (Gennadius). J Basic Appl Zool 65:17–20. https://doi.org/10.1016/j.jobaz.2012.01.003
Mohammed IU, Abarshi MM, Muli B, Hillocks RJ, Maruthi MN (2012) The symptom and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Virol Adv. https://doi.org/10.1155/2012/795697
Muhindo H, Yasenge S, Casinga C, Songbo M, Dhed’a B, Alicai T, Pita J, Monde G (2020) Incidence, severity and distribution of cassava brown streak disease in North-eastern democratic republic of Congo. Cogent Food Agric 6:1. https://doi.org/10.1080/23311932.2020.1789422
Mwila N, Rubaihayo PR, Kyamanywa S, Odong TL, Nuwamanya E, Mwala M, Agbahoungba S, Badji A (2017) Biochemical factors associated with cassava resistance to whitefly infestation. Afr Crop Sci J 25:365–385. https://doi.org/10.4314/acsj.v25i3.9
Ntawuruhunga P, Ssemakula G, Ojulong H, Bua A, Ragama P, Kanobe C, Whyte J (2006) Evaluation of advanced cassava genotypes in Uganda. Afr Crop Sci J 14:15–27
Obiero HM (2004) Accelerated cassava multiplication and distribution of improved planting materials in western Kenya. In: Emergency program to combat the cassava mosaic disease pandemic in east and central Africa. Proceedings of the Fifth regional stakeholders meeting, Bukoba, Tanzania, pp. 10-12
Omongo CA, Colvin J, Sserubombwe W, Alicai T, Baguma Y, Bua A, Legg JP, Gibson RW (2004) Host-plant resistance to African Bemisia tabaci in local landraces and improved cassava mosaic disease resistant genotypes in Uganda. In: The 6th international scientific meeting of the cassava biotechnology network. Cali, Colombia, p. 84.
Shibuya T, Hirai N, Sakamoto Y, Endo R (2010) Preference of sweetpotato whitefly adults to cucumber seedlings grown under two different light sources. Horttech 20:873–876. https://doi.org/10.21273/HORTTECH.20.5.873
Shirima RR, Maeda DG, Kanju EE, Tumwegamire S, Ceasar G, Mushi E, Sichalwe C, Mtunda K, Mkamilo G, Legg JP (2019) Assessing the degeneration of cassava under high-virus inoculum conditions in coastal Tanzania. Plant Dis 103:2652–2664. https://doi.org/10.1094/PDIS-05-18-0750-RE
Sseruwagi P, Legg JP, Maruthi MN, Colvin J, Rey MEC, Brown JK (2005) Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations and the presence of the B biotype and a non-B biotype that can induce silverleaf symptoms in squash. Uganda Ann Appl Biol 147:253–265. https://doi.org/10.1111/j.1744-7348.2005.0026.x
Storey HH, Nichols RFW (1938) Studies of the mosaic diseases of cassava. Ann Appl Biol 25:790–806. https://doi.org/10.1111/j.1744-7348.1938.tb02354.x
Tairo F, Sseruwagi P, Kayuki C, Bachwenkizi H, Fute T, Mark D, Lipala R, Mushi L, Erasto J, Kileo S, Mkangwa R, Mlegi V, Lupembe M, Ngazi V, Saggaf M, Kidulile C, Lwabulala D, Rajabu C, Ndunguru J (2019) The cassava diagnostics project: a review of 10 years of research. Tanzania Agricultural Research Institute (TARI), Mikocheni. pp. 29–42
Tembo M, Mataa M, Legg J, Chikoti PC, Ntawuruhunga P (2017) Cassava mosaic disease: incidence and yield performance of cassava cultivars in Zambia. J Plant Pathol 99:681–689. https://doi.org/10.4454/JPP.V99I3.3955
Thresh JM, Cooter RJ (2005) Strategies for controlling cassava mosaic virus disease in Africa. Plant Pathol 54:587–614. https://doi.org/10.1111/j.1365-3059.2005.01282.x
Thresh JM, Otim-Nape GW, Legg JP, Fargette D (1997) African cassava mosaic disease: the magnitude of the problem. Afr J Root Tuber Crops 2:13–19
Acknowledgements
The African Union Commission (AUC) funded this work under grant numbers AURG/2/141 and AURG II-1-060-2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mkamilo, G., Kimata, B., Masinde, E.A. et al. Impact of viral diseases and whiteflies on the yield and quality of cassava. J Plant Dis Prot 131, 959–970 (2024). https://doi.org/10.1007/s41348-024-00903-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-024-00903-3