Abstract
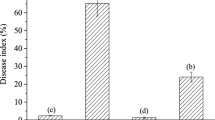
Black pepper-associated endophytic Pseudomonas putida BP25 displayed volatile-mediated antagonism against rice blast fungus Magnaporthe oryzae 1637. The major chemical fraction identified in the volatiles of Pseudomonas putida BP25 was 2-methylpyrazine and 2-ethyl-3,6-dimethylpyrazine; both of them inhibited all developmental stages such as conidial germination, mycelial growth, and sporulation of M. oryzae. To validate the antifungal activity of 2-methylpyrazine and 2-ethyl-3,6-dimethylpyrazine on blast disease, in planta experiments were conducted on rice seeds and seedlings. Blast disease incidence and disease severity on volatile-treated seedlings were significantly reduced as compared to mock. Seedlings that emerged from volatile-exposed seeds were analyzed for expression of candidate defense gene, OsPAD4, OsEDS1, OsPDF2.2, OsPR3, and OsPR1.1 by quantitative real-time PCR (qPCR). Seedlings exposed to volatiles showed significant induction of the OsPAD4 gene with both 2-ethyl-3,6-dimethylpyrazine and 2-methylpyrazine. Direct antifungal activity of pyrazine coupled with its defense elicitation capability can be harnessed for rice seed disinfection to ensure healthy, vigorous, and disease-free transplants.






Similar content being viewed by others
References
Agisha VN, Kumar A, Eapen SJ, Sheoran N, Suseelabhai R (2019) Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci Technol 29:1069–1089
Aravind R, Kumar A, Eapen SJ, Ramana KV (2009) Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol 48:58–64
Ashajyothi M, Kumar A, Sheoran N, Ganeshan P, Gogoi R, Subbaiyan G, Bhattacharya R (2019) Black pepper (Piper nigrum L.) associated endophytic Pseudomonas putida BP25 alters root phenotype and induces defense in rice (Oryza sativa L.) against blast disease incited by Magnaporthe oryzae. Biol Control 143:104181
Audrain B, Farag MA, Ryu CM, Ghigo JM (2015) Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol Rev 39:222–233
Bailly A, Weisskopf L (2012) The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal Behav 7:79–85
Blom D, Fabbri C, Connor E, Schiestl F, Klauser D, Boller T, Eberl L, Weisskopf L (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058
Braña AF, Rodríguez M, Pahari P, Rohr J, García LA, Blanco G (2014) Activation and silencing of secondary metabolites in Streptomyces albus and Streptomyces lividans after transformation with cosmids containing the thienamycin gene cluster from Streptomyces cattleya. Arch Microbiol 196:345–355
Brilli F, Loreto F, Baccelli I (2019) Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front Plant Sci 10:264
Callaway E (2016) Devastating wheat fungus appears in Asia for first time. Nat News 532:421
Campos VP, Pinho RSCd, Freire ES (2010) Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciência e Agrotecnologia 34:525–535
Cavara F (1891) Fungi Longobardiae exsiccati sive mycetum specimina in Longobardia collecta, exsiccata et speciebus novis vel criticis, iconibus illustrata. Pugillus I 18
Ceresini PC, Castroagudin VL, Rodrigues FA, Rios JA, Aucique-Pérez CE, Moreira SI, Alves E, Croll D, Maciel JLN (2018) Wheat blast: past, present, and future. Annu Rev Phytopathol 56:427–456
Chen H, Xiao X, Wang J, Wu L, Zheng Z, Yu Z (2008) Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol Lett 30:919–923
Chernin L, Toklikishvili N, Ovadis M, Kim S, Ben-Ari J, Khmel I, Vainstein A (2011) Quorum-sensing quenching by rhizobacterial volatiles. Environ Microbiol Rep 3:698–704
Choi HK, Song GC, Yi HS, Ryu CM (2014) Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J Chem Ecol 40:882–892
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Effmert U, Kalderás J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38:665–703
FAO (2015) World food situation. FAO cereal supply and demand brief. Food and Agriculture Organization, Rome. http://www.fao.org/worldfoodsituation/csdb/en/.
Filippi MCC, Da Silva GB, Silva-Lobo VL, Côrtes MVC, Moraes AJG, Prabhu AS (2011) Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biol Control 58:160–166
Gnanamanickam SS, Mew TW (1992) Biological control of blast disease of rice (Oryza sativa L.) with antagonistic bacteria and its mediation by a Pseudomonas antibiotic. Jpn J Phytopathol 58:380–385
Herrington P, Craig J, Chea C, Sheridan J (1985) Inhibition of spore germination by volatiles from Streptomyces griseoruber. Soil Biol Biochem 17:897–898
Herrington P, Craig J, Sheridan J (1987) Methyl vinyl ketone: a volatile fungistatic inhibitor from Streptomyces griseoruber. Soil Biol Biochem 19:509–512
Horiuchi JI, Muroi A, Takabayashi J, Nishioka T (2007) Exposing Arabidopsis seedlings to borneol and bornyl acetate affects root growth: specificity due to the chemical and optical structures of the compounds. J Plant Interact 2:101–104
Huang CJ, Tsay JF, Chang SY, Yang HP, Wu WS, Chen CY (2012) Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci 68:1306–1310
Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol 14:84
Islam MT, Kim KH, Choi J (2019) Wheat blast in Bangladesh: the current situation and future impacts. Plant Pathol J 35:1
Kai M, Piechulla B (2010) Impact of volatiles of the rhizobacteria Serratia odorifera on the moss Physcomitrella patens. Plant Signal Behav 5:444–446
Kai M, Crespo E, Cristescu SM, Harren FJM, Francke W, Piechulla B (2010) Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl Microbiol Biotechnol 88(4):965–976
Ke Y, Liu H, Li X, Xiao J, Wang S (2014) Rice OsPAD4 functions differently from Arabidopsis AtPAD4 in host-pathogen interactions. Plant J 78:619–631
Kwon YS, Ryu CM, Lee S, Park HB, Han KS, Lee JH, Lee K, Chung WS, Jeong MJ, Kim HK (2010) Proteome analysis of Arabidopsis seedlings exposed to bacterial volatiles. Planta 232:1355–1370
Lee B, Farag MA, Park HB, Kloepper JW, Lee SH, Ryu CM (2012) Induced resistance by a long-chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE 7:48744
Malaker PK, Barma N, Tiwary T, Collis WJ, Duveiller E, Singh K, Joshi AK, Singh RP, Braun HJ, Peterson GL (2016) First report of wheat blast caused by Magnaporthe oryzae pathotype triticum in Bangladesh. Plant Dis 100:2330
Munjal V, Nadakkakath AV, Sheoran N, Kundu A, Venugopal V, Subaharan K, Rajamma S, Eapen SJ, Kumar A (2016) Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol Control 92:66–76
Munjal V, Kumar A, Sheoran N, Nadakkakath AV, Eapen SJ (2017) Molecular basis of endophytic Bacillus megaterium-induced growth promotion in Arabidopsis thaliana: revelation by microarray-based gene expression analysis. J Plant Growth Regul 36:118–130
Ou S (1980) Pathogen variability and host resistance in rice blast disease. Annu Rev Phytopathol 18:167–187
Prabhu A, Filippi M (1993) Seed treatment with pyroquilon for the control of leaf blast in Brazilian upland rice. Int J Pest Manag 39:347–353
Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate-and jasmonate-dependent signaling. Mol Plant Microbe Interact 20:492–499
Rajashekara H, Ellur RK, Khanna A, Nagarajan M, Gopalakrishnan S, Singh A, Sharma P, Sharma T, Singh U (2014) Inheritance of blast resistance and its allelic relationship with five major R genes in a rice landrace “Vanasurya”. Indian Phytopathol. 67:365–369
Rajini K, Aparna P, Sasikala C, Ramana CV (2011) Microbial metabolism of pyrazines. Crit Rev Microbiol 37:99–112
Ryan RP, Dow JM (2008) Diffusible signals and interspecies communication in bacteria. Microbiology 154:1845–1858
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Sahu KP (2015) Metagenomic analysis of rice phyllospheric bacterial communities in relation to blast disease. Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi. https://krishikosh.egranth.ac.in/handle/1/5810021259
Sharifi R, Ryu CM (2016) Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to Arabidopsis seedlings for eliciting induced resistance, or both? Front Microbiol 7:196
Sheoran N, Nadakkakath AV, Munjal V, Kundu A, Subaharan K, Venugopal V, Rajamma S, Eapen SJ, Kumar A (2015) Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol Res 173:66–78
Sheoran N, Kumar A, Munjal V, Nadakkakath AV, Eapen SJ (2016) Pseudomonas putida BP25 alters root phenotype and triggers salicylic acid signaling as a feedback loop in regulating endophytic colonization in Arabidopsis thaliana. Physiol Mol Plant Pathol 93:99–111
Shimoi S, Inoue K, Kitagawa H, Yamasaki M, Tsushima S, Park P, Ikeda K (2010) Biological control for rice blast disease by employing detachment action with gelatinolytic bacteria. Biol Control 55:85–91
Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, Bais H (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14:130
Wenke K, Wanke D, Kilian J, Berendzen K, Harter K, Piechulla B (2012) Volatiles of two growth-inhibiting rhizobacteria commonly engage AtWRKY18 function. Plant J 70:445–459
Wilson RA, Talbot NJ (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7:185
Acknowledgements
Authors are thankful to Director, ICAR-Indian Agricultural Research Institute, New Delhi, and PG School, ICAR-Indian Agricultural Research Institute, New Delhi, for providing financial support and research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A., Kumar, A., Sheoran, N. et al. Antifungal and defense elicitor activities of pyrazines identified in endophytic Pseudomonas putida BP25 against fungal blast incited by Magnaporthe oryzae in rice. J Plant Dis Prot 128, 261–272 (2021). https://doi.org/10.1007/s41348-020-00373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00373-3