Abstract
Sea pens are some of the most important structural species in soft bottom benthic communities. Pteroeides spinosum is a shallow-water pennatulacean (Cnidaria: Octocorallia: Pennatulacea) habiting NE Atlantic-Mediterranean bottoms. In this study, sixteen colonies of Pteroeides spinosum collected from NW Mediterranean Sea (Cap de Creus, Spain) at 99–106 m depth in July (2012), were used to acquire information of their reproductive biology, and to enable a comparison with other pennatulaceans. Our colonies of Pteroeides spinosum are gonochoric showing a bimodal oocyte frequency distribution and an unimodal spermatocyst diameter distribution. All polyp leaves contained oocytes and spermatocysts in different size classes, with those small and translucent spermatocysts/ oocytes (size-class I) often found near the base or lower end of the gastrovascular cavities of polyps. The largest observed diameter was 668 μm for spermatocysts and 687 μm for oocytes. Mean potential relative fecundity (PRF) ranged between 7–17 oocytes per polyp. The effective reproductive effort (ERE) reached a value of ca. 30,000 large oocytes per colony. As was observed for all pennatulaceans previously studied, no sign of hermaphroditism or internal fertilization was detected in our colonies of Pteroeides spinosum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite all efforts to improve our knowledge of octocoral biology (see Orejas et al. 2002; Page and Lasker 2012; Coelho and Lasker 2014; among others) many groups remain poorly studied from a reproductive point of view. Information on the reproductive biology of the species is essential to understanding population dynamics and potential responses to disturbances (Servetto et al. 2013).
This lack of knowledge limits our current ability to assess the survival potential of species, as well as other biological processes, such as the resilience of the population after a reduction in the number of individuals due to natural or anthropogenic disturbances. Determining the reproductive features of a species (such as reproductive effort, reproductive cycle, fecundity, sex ratio, spawning events, etc.) not only improves our knowledge of the particular species, but also provide us with information that constitutes a useful tool for designing conservation plans (Connor et al. 2003; Davies et al. 2017), as well as to infer the vulnerability of that species, which in turn will indirectly affect a set of associated species that coexist with it (Roberts et al. 2006).
In this sense, octocorals form essential habitats that host an important reserve of associated fauna (Baillon et al. 2012; Clippele et al. 2015). Banks or gardens of these bioconstructor organisms are often referred to as biodiversity hot spots, similar to tropical coral reefs (Buhl-Mortensen et al. 2010) with a high vulnerability to natural or anthropogenic disturbances (Roberts 2002; Carney 2005).
Among the octocorals, sea pens (Octocorallia: Pennatulacea) seem to be the most specialized group, with significant morphological adaptations, such as a muscular peduncle that serves as an anchoring system on muddy and sandy bottoms (Kükenthal 1915; Williams 2011), where they frequently act as bioconstructors of a benthic tree-dimensional landscape on which a rich fauna is housed (Baillon et al. 2012; Chimienti et al. 2018). This important ecological role has been internationally recognized by including sea pen beds and their associated megafauna in the OSPAR list of threatened and/or declining species and habitats (Jones et al. 2000; Curd 2010).
Our knowledge about the reproductive characteristics of sea pens has increased over the last 20 years (Eckelbarger et al. 1998; Tremblay et al. 2004; Lopes et al. 2012; Servetto and Sahade 2016; Hamel et al. 2020; Couillard et al. 2021; among others). Thanks to these previous studies we know that pennatulaceans are exclusively gonochoric and broadcast spawning organisms, and to date, no solid evidence of internal larval development or parthenogenesis events have been found (e.g. Baillon et al. 2014; Hamel et al. 2020; Couillard et al. 2021).
Sea pen species with a high fecundity have been described, producing up to 200,000 oocytes per colony in one season (Chia and Crawford 1973). Large oocytes up to 1,200 µm in diameter have been found (Pires et al. 2009), which after the spawning event and external fertilization process, produce lecithotrophic planula larvae that actively swim before settling and metamorphosing when suitable substrate is detected (Chia and Crawford 1973).
Various reproductive temporal patterns have been reported in sea pens. On the one hand, shallow-water pennatulaceans seem mainly to follow an annual cycle in which oocyte development takes at least 12 months to complete and for the oocytes to be released into the water column, in a one single spawn event (Tremblay et al. 2004; Edwards and Moore 2008). As occurred frequently in octocorals (see Simpson 2009), this prolonged cycle of oocyte development commonly involves the presence of a bimodal (or trimodal, see Edwards and Moore 2008, 2009; Couillard et al. 2021) frequency distribution of oocyte sizes, with a constant pool of immature oocytes throughout the year, and another category involving a smaller quantity of larger-sized oocytes developing over time to the maximum sizes at which they will be emitted (Edwards and Moore 2009; Servetto and Sahade 2016). On the other hand, some species have been reported to exhibit a “continuous” or “non-seasonal” reproductive cycle (Rice et al. 1992; Eckelbarger et al. 1998; Pires et al. 2009).
In short, despite these efforts, most of the sea pens species (around 92%) remain unexplored from a reproductive point of view (Baillon et al. 2014; Servetto and Sahade 2016), some of the reproductive traits analyzed vary among pennatulacean species (Baillon et al. 2015) (e.g. continuous or seasonal cycle) and there is a complex methodology (e.g. fecundity indices) difficult to apply to the whole set of diverse morphologies known in sea pens (see Williams 1995). In this early stage of knowledge, any new reproductive information will expand our comprehension about these sometimes inaccessible organisms.
The genus Pteroeides Herklots, 1858 presents a wide distribution, from the Eastern Atlantic and Mediterranean Sea to the West Indo-Pacific, and it is known to inhabit a bathymetric range from 15 to 400 m in depth (Williams 1995, 2011; García-Cárdenas and López-González 2022). Colonies of Pteroeides are mostly stout and feather-like, with bilateral symmetry in the rachis throughout, the axis is present throughout the colony and polyp leaves are well-developed and rigid due to the presence of one to many supporting rays composed of long needle-like sclerites (Williams 1995).
Pteroeides spinosum (Ellis and Solander, 1786) has been mainly reported from the Mediterranean Sea (Herklots 1858; Gray 1870; Kükenthal 1915; Pax and Müller 1953, 1955, 1959; Rossi 1971; Gili and Pagès 1987; Abdelsalam 2014; Porporato et al. 2014; Topçu and Öztürk 2015), but also in the Northeastern Atlantic Ocean (Madeira island, Ocaña and Wirtz 2007; Tangier bay, Marshall and Fowler 1887), in a bathymetric range from 20 to 295 m in depth.
Moreover, there are no published studies on its reproductive characteristics, perhaps due to the difficult access to these colonies. Only a cool-water, indeterminate population of Pteroeides studied by Duncan (1998, unpub. MSc thesis) have been recorded in the literature. In the present contribution, we provide, at the first time, some observations on the reproductive stage of a NW Mediterranean Sea population of the type species Pt. spinosum.
Materials and Methods
Sampling and Sex Determination
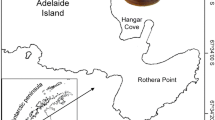
The colonies of Pteroeides spinosum here studied were collected from NW Mediterranean Sea (Cap de Creus, Spain), using a Rauschert dredge (RD) during the oceanographic campaign INDEMARES VI, carried out between June and July 2012 (Fig. 1). The material analysed in this study was collected at the stations 131 (42º 22,199’N 3º 16,887’E) and 132 (42º 20,011’N 3º 20,085’E), between 99 and 106 m depth, on July 8, 2012. A total of sixteen colonies were collected. The specimens were sorted and labelled on board. The colonies were fixed in hexamethylenetetramine-buffered 4% formalin-seawater. After the fixation period (a week), all colonies were preserved in 70% ethanol. Sixteen colonies were examined and total colony length was measured using ImageJ 1.38 × software. Colony sizes ranged between 73 to 138.5 mm in total length, and were a priori grouped into two size classes (< 100 mm, and > 100 mm of total length) in order to check possible morphological and reproductive differences.
The terminology used in colony anatomy mainly follows Bayer et al. (1983). The studied material is deposited in the Zoological Museum of Barcelona (Spain) and in the collection of the research group Biodiversidad y Ecología Acuática at the University of Seville (BECA).
The polyps were dissected and their sexual products extracted for examination under a Motic SMZ-140 stereo microscope. Feret diameter (fD) measures (Flint et al. 2007) of oocytes and spermatocysts were obtained using a calibrated eyepiece. Initial sex determination was done on the observation of gametes (from one or two fragments of different polyp leaves) in lactic acid, and corroborated along the counting phase of the study. Oocytes and spermatocysts were readily distinguishable: oocytes had a relatively large nucleus with nucleolus, and a bright yellow coloration; spermatocysts had a granulate aspect with multiple sperm heads and a translucent appearance. The deviation of a 1:1 sex ratio was assessed using a chi-squared test (Zar 1984).
Intracolonial Variation
The total length of rachis of male and female colonies was divided into three zones of similar length, namely proximal, medial and distal (Fig. 2), to evaluate possible differences in reproductive traits among zones, following previous studies on reproduction in octocorals (see Orejas et al. 2002; Soong 2005; Edwards and Moore 2008; among others).
Details of a male (A) and female (B) colony of Pteroeides spinosum showing the considered zones in the rachis; C details of a polyp leaf showing the arrangement of autozooids (az) and the prominent groups of spicules (rays) (e); D a fragment of a polyp leaf showing the retracted anthocodia of autozooids (ant), and gastrovascular cavities (gc); E–F, MO images
On the one hand, intracolonial variation in macro-morphological traits, such as polyp leaf lengths and number of autozooids per leaf, were analysed. On the other hand, the mean value of the oocytes and spermatocysts number and size (fD) from two polyp leaves in each zone was registered.
In summary, the effects and interactions of the factors “size” and “zone” were evaluated in the following variables: (1) mean polyp leaf lengths; (2) mean polyps per leaf; (3) mean gametes per leaf; and (4) mean gametes per polyp, also called potential relative fecundity (PRF, Baillon et al. 2014) [note that “gametes” includes here oocytes and spermatocysts]. Possible variations within and among colonies were tested using homogeneity of variance (Levene) and ANOVA tests.
Gamete Sizes
Different spermatocyst/ oocyte sizes were classified into two size categories, according to the diameter gamete distribution and following previous studies in order to ensure further comparisons (e.g. Mladenov and Brady 1987; McClary and Mladenov 1989; Duncan 1998; Servetto and Sahade 2016): size-class I or small gametes (< 300 μm) and size-class II or large gametes (> 300 μm) (see Discussion). Size-class I is a priori considered immature stage; and size-class II is considered close to gamete mature stages, which would be released at the spawning event. The mean number and percentage of spermatocysts/ oocytes in each size class was obtained at zone and at colony level. To determinate the maturity stage of a given colony, we identified the leading cohort of gametes (LCO, leading cohort of oocytes; LCS, leading cohort of spermatocysts), using the ten largest oocytes/ spermatocysts in each one, following previous studies (Couillard et al. 2021).
Gamete-diameter Frequency Distribution
Data of spermatocyst and oocyte measurements were used to construct diameter-frequency histograms, taking into account the factors “size” and “zone” of colony per sex. A cluster analysis for K-means to clearly determine the “hidden” groups in the point cloud was driven (Laverick et al. 2017; Nurdin et al. 2019). A robust one-way Analysis of Variance (ANOVA) to verify statistically differences among groups was applied.
Fecundity
Potential relative fecundity (PRF) defined as the total number of oocytes/ spermatocysts per polyp was estimated irrespective of their size (or maturity stage) by dividing the total number of oocytes/ spermatocysts per polyp leaf by the number polyps on that polyp leaf (Baillon et al. 2014, 2015). Here, this PRF was obtaining using the two gamete size classes. As the reproductive cycle of Pteroeides spinosum is unknown, this value must be considered with caution to further validation (see Discussion).
Statistical Analysis
Colony zones, polyp leaves and spermatocyst/ oocyte dimensions were measured using ImageJ 1.38 × software. Normality was tested using Shapiro–Wilk test, and homogeneity of variances was examined using Levene's test (Servetto and Sahade 2016). When an assumption of normality was not confirmed (e.g. the bimodal distribution of oocytes) and variances were not homogeneous, a cluster analysis for K-means was driven followed by a robust one-way Analysis of Variance (ANOVA, Welch and Brown-Forsythe tests), and then multiple range tests (such as HDS Tukey) were used post hoc (Servetto and Sahade 2016). Statistical data analyses were performed using IBM SPSS Statistics v22 (SPSS INC., Chicago, USA).
Nomenclatural Remark
There is still some confusion about what the appropriate binomen and authorship should be considered currently valid for the species here studied. Many publications used Pteroeides griseum (Bohadsch 1761) as the accepted name, while others cited it as Pteroeides spinosum (Ellis, 1764). As noted by Williams (1995: 130), the binomen Penna grisea Bohadsch, 1761 is not admissible since Bohadsch (1761) was suppressed by ICZN (1944). However, there is also some confusion regarding to who and when the specific epithet spinosa appeared in the literature, because Ellis (1764) did no mentioned the name spinosa in his paper. The name spinosa as specific epithet for this species appeared for the first time in Ellis and Solander (1786) (see Suppl. Mat. Doc.1 for a detailed review of the nomenclatural vicissitudes on the binomen and taxonomic authorities for this species). In summary, the valid name for the species here studied is considered Pteroeides spinosum (Ellis and Solander 1786).
Results
All our Pteroeides spinosum colonies were in reproductive stage. In the live state, females with oocytes in advanced state of maturation are clearly differentiated by pale apricot to bright orange colour as their oocytes are visible through the lateral sides of the polyp leaves by transparency (see Fig. 2B), while in male colonies lateral sides of the polyp leaves are cream to yellow (not shown). A part of this gametogenic structure of oocytes and spermatic cysts is the typical already observed in other octocoral species (Fig. 2E, F). Specimens examined had no significant deviation from a sex ratio of 1:1 (comprising 7 male and 9 female colonies) (p = 0.617; χ2 = 0.25; df = 1).
Differences Among Colony Size Classes
Although two size classes in each sex were differentiated [small and large males (86–99 mm, 106–107 mm, p < 0.05; χ2 = 10.32; df = 1); small and large females (73–91 mm, 101–138 mm p < 0.05; χ2 = 19.87; df = 1) (Table S1)], some features analyzed did not appear to differ between both size classes, such as the number of polyp leaves per colony (small: 34–47 polyp leaves; large: 36–60 polyp leaves, Table S1), the mean polyp leaf lengths or the mean polyps per polyp leaf. Only the mean oocytes per polyp leaf (Table S2) showed significant differences between certain small and large colonies (p < 0.05; χ2 = 505.74; df = 1). The highest value of spermatocysts/ oocytes per polyp leaf was reached in larger colonies (1,805 spermatocysts per leaf and 3,829 oocytes per leaf) (Table S2).
Statistical analyses supported the idea that two dependent variables (mean polyp leaf length, mean polyps per leaf) showed no significant variation between colony sizes. However, the other variables (mean oocytes per polyp leaf and mean oocytes per polyp) were useful to differentiate among some of the small and large colonies (p = 0.018, df = 8, Brown-Forsythe; p = 0.001, df = 8, Brown-Forsythe). These colonies with significant differences were the smaller females (♀1, ♀4, and ♀5) with the lowest values of oocytes per polyp leaf and per polyp, and two larger females (♀7 and ♀8) with the highest mean oocytes per polyp (p = 0.001, df = 8, Brown-Forsythe and post hoc HSD Tukey test).
Intracolonial Variation
Contrary to the variation among size classes, the variation within the colony or comparisons between zones, showed differences strongly supported by the majority of studied features, regardless of the size and sex of the colonies.
The medial zone of the rachis presented the lowest mean number of polyp leaves (♂ 13 ± 2, 9 ± 2, 16 ± 3; ♀ 13 ± 3, 12 ± 3 and 16 ± 3 polyp leaves for proximal, medial and distal zones, respectively), the largest polyp leaf lengths (♂ p = 0.043; χ2 = 4.06; df = 1; ♀ p = 0.001; χ2 = 9.98; df = 1), the highest mean polyps per leaf and the highest mean oocytes per leaf (p < 0.05; χ2 = 853.43; df = 1) (Table S2). In summary, the mean number of oocytes (and spermatocysts) per polyp leaf was higher in the medial zone and decreased towards the proximal and distal ends of the rachis.
In general, the lowest means were found in polyp leaves from the proximal zone of rachis, although with little variation with respect to the distal zone, the medial zone having the highest means of oocytes per polyp leaf and PRF values (Table S2).
Gamete Sizes
All the polyp leaves observed contained oocytes and spermatocysts in different sizes probably as result of different stages of maturation (as size-classes in this study) (Fig. 2). Those more opaque and large spermatocysts/ oocytes (size-class II) were frequently found at the base and middle part of the mesenterial filaments of polyps, near the mouth. While those small and translucent spermatocysts/ oocytes (size-class I) were most often found near the lower end of the gastrovascular cavities of polyps (anchored to the lower end of filaments), embedded in the axillary area of the polyp leaves (Fig. 2).
In male colonies, a total of 27,553 spermatocysts were counted (15,499 and 12,054 cysts in small and large colonies, respectively), with a size range between 37.50 and 668.20 μm (Table 1). Regardless of colony size, the highest number of spermatocysts was in size-class I (19,954 cysts) and a few in size-class II (7,599 cysts). The medial-distal zones presented the highest percentage of spermatocysts in size-class II, while the proximal zone showed the highest percentage of spermatocysts in size-class I, the lowest percentage in size-class II, and no spermatocysts were found > 500 μm (Tables 1 and S3).
In female colonies, a total of 57,850 oocytes were counted (21,719 and 36,131 oocytes in small and large colonies, respectively), with a size range between 25 and 687.50 μm (Table 1). The largest number of oocytes was in size-class I (37,875 oocytes), followed by size-class II (19,975 oocytes). As with spermatocysts, the proximal zone had the highest percentage of oocytes in size-class I, and the medial-distal zones had the highest percentage of oocytes in size-class II. In this case a low percentage of large oocytes was found in the proximal zone (Tables 1 and S3).
Diameter Frequency Distribution
Spermatocyst and oocyte diameter-frequency distributions of all Pteroeides spinosum colonies are shown combined in Fig. 3 and individually in Figs. 4 and 5. Spermatocyst distributions (Fig. 3, upper row) revealed a unique large cohort of small cysts, ranged between 50 and 300 μm (mainly formed by spermatocysts in size-class I). This distribution was similar among the three zones within the colony (i.e. there was synchrony), but the highest values were reached in the medial zone. This spermatocyst distribution was observed in the seven male individual colonies (Fig. 4).
Diameter frequency distributions of spermatocysts and oocytes in three colony zones of all Pteroeides spinosum colonies combined: K1, mean diameter of small oocytes (size-class I); K2 mean diameter of large oocytes (size-class II). The statistical analyses shown correspond to the three zones of female colonies combined
On the other hand, oocytes showed a well-defined bimodal diameter-frequency distribution (Fig. 3, bottom row), with a more numerous younger oocyte cohort (< 250 μm, in size-class I), and another large oocyte cohort (> 250 μm, in size class II) that included the highest diameter but with the lowest frequencies. This bimodal distribution is maintained in all zones, showing a clear within-colony synchronization.
In female colonies, the cluster analysis for K-means statistically supported these two cohorts (K = 2; K1 mean of small oocytes with a mean of 117.65 μm and 38,551 units; and K2 mean of large oocytes with a mean of 454.37 μm and 18,751 units) in the three zones (p < 0.00; Welch and Brown-Forsythe). It can be seen that Pt. spinosum females presented two overlapping oocytes cohorts with synchronized harmony within the colony. The second cohort would include oocytes at advanced stages of maturity. Individually analysed female colonies clearly showed this bimodal distribution, with no differences between size-class colonies (Fig. 5). The maturity stage of female colonies of Pt. spinosum based on the mean diameter of the largest oocytes (i.e., leading cohort), was similar between the size classes, and ranged between 400–700 μm regardless the total number of oocytes examined (Table 1 and Fig. 6). The relationship between the mean number of spermatocysts and oocytes and the colony height was positive and more pronounced in male than female colonies (R2 0.6599 and R2 0.3421, respectively); similarly, the relationship between leading cohort oocyte size (LCO) and the total colony length was also positive but less pronounced (R2 0.1033) (Fig. 7).
Mean potential relative fecundity (PRF) was estimated to be 7 oocytes per polyp at the proximal and distal zones, and 10 oocytes per polyp at the medial zone in the smaller females; while in larger females, 12–13 oocytes per polyp were found at the proximal and distal zones, and 17 oocytes per polyp at the medial (Table S2). Although the higher values were presented at the medial zone of the rachis, they did not show significant differences with the other zones (p > 0.05, HSD Tukey). The relationship between PRF and the total colony length using individual colonies, although positive, did not show a clear lineal increase (Fig. 8).
The potential reproductive effort (PRE) of our colonies increased from small to large colonies, and from lower to upper zones (medial and distal) of the rachis in all the colonies examined (Table 2). In the smaller males, PRE was 19,502 ± 1,228 spermatocysts per colony (ranging from 6,611–32,364); and in larger males PRE was 35,646 ± 1,530 spermatocysts per colony (ranging from 24,308–46,645) (Table 2). Measures of PRE taken within colony, showed the lowest mean values at the proximal zone (1,572 and 10,294 spermatocysts per zone for small and large males, respectively).
In the smaller females, PRE was 27,379 ± 2,737 oocytes per colony (ranging from 8,402–53,140), and in the larger females PRE was 73,114 ± 7,090 oocytes per colony (ranging from 26,445–110,283) (Table 2). Within the colony, in both colonial sizes, the highest mean PRE values were at the medial zone (16,621 and 42,802 oocytes per zone, respectively) (p < 0.05; χ2 = 93,683.8; df = 1), the lowest PRE values always being found at the proximal zone of the rachis (Tables 2 and S4).
The effective reproductive effort (ERE) increased from the smaller to the larger colonies, and was strongly lower at the proximal zone of the rachis in all colonies examined. In males, ERE was 5,008 ± 801 and 9,946 ± 1,448 large spermatocysts per colony for small and large colonies, respectively. Measures of ERE taken within the colony again showed the lowest values at the proximal zone (proximal < medial ≈ distal) (Table 2). In females, ERE was 6,828 ± 1,417 and 29,876 ± 6,871 large oocytes per colony for small and large colonies, respectively. Within the colony, in both colonial sizes the medial zone had significantly the highest ERE values (4,973 and 19,142 oocytes per zone, respectively) (p < 0.05; χ2 = 24,031; df = 1) (Tables 2 and S4).
In short, one of our larger Pt. spinosum females might contain up to 110,000 oocytes, but maybe only 27% (~ 30,000) reach a mature stage.
Discussion
Despite an annual sampling programme was not feasible from a logistic point of view for this study, especially difficult when studying deep sea benthic species (as mesophotic depths), relevant information from a single or limited number of samples of sea pen populations can contributes to improving our current knowledge about the reproductive biology of these species (see Chia and Crawford 1973; Eckelbarger et al. 1998).
Many of the reproductive features observed in our colonies of Pteroeides spinosum are shared with the population of Pteroeides sp. studied by Duncan (1998) from Fiorland (New Zealand), as well other Mediterranean pennatulaceans (as Veretillum cynomorium, see Lopes et al. 2012) and alcyonaceans such as Eunicella singularis, Paramuricea clavata, Alcyonium acaule or Alcyonium coralloides (see Theodor 1967; Weinberg and Weinberg 1979; Coma et al. 1995a, b; Fiorillo et al. 2013; Quintanilla et al. 2013). All of them had spermatocysts and oocytes at different sizes corresponding to different stages of maturity, as well as a distinguishable bi- or tri-modal oocyte size distribution and a high reproductive effort at colony level. In our colonies no sign of hermaphroditism or internal fertilization was observed, as is also assumed in the rest of pennatulaceans (e.g. Baillon et al. 2014, 2015).
In population studies the importance of spatial distribution and the proportion of male and female colonies is well known (see Simpson 2009). Certain authors have reported that the highest fertilization percentage occurs in a population with both sexes (Benayahu and Loya 1983; Brazeau and Lasker 1992; Coffroth and Lasker 1998) and with a ratio close to 1:1 (Coma et al. 1995a, b; Pires et al. 2009). The colonies examined in the present study displayed gonochorism and equality of sex-ratio, in line with most pennatulacean species studied (Rice et al. 1992; Edwards and Moore 2008; Baillon et al. 2014, 2015; Hamel et al. 2020; Couillard et al. 2021).
Influence of Colony Size
Considering that further statistical analyses are needed (see Hamel et al. 2020; Couillard et al. 2021), and although a priori different size classes were observed in our Pteroeides spinosum specimens collected, most features analyzed here did not support significant differences between the small and large size classes considered (Table S2), all colonies being in reproductive stage (Figs. 6 and 7). However, two features, mean oocytes per polyp leaf and mean oocytes per polyp (fecundity), enabled differentiation between some smaller colonies, with the lowest values. In this sense, the relationship between fecundity (PRF) and the colony height examined using individual colonies data (Fig. 8) was positive although lower pronounced than in other species (see Hamel et al. 2020 for Umbellula encrinus). This influence of colony size on the reproductive effort was also reported in other sea pens (see Baillon et al. 2015 for Halipteris finmarchica). Perhaps a considerable increase of Pt. spinosum colonies would offer a more accuracy relationship between PRF-colony height factors.
In our colonies, it was not possible to determine the number of large oocytes per polyp (known as effective relative fecundity, ERF) because the elongated gastrovascular cavities of autozooids are embedded within large and fleshy polyp leaves (see also Duncan 1998). This implies, in this case, that fecundity can only be estimated at the polyp level as PRF (Soong 2005, see Discussion).
Although spawning time and whether there is a single or more than one spawning events are also unknown, we offer a speculative estimate at colony level using the potential reproductive effort (PRE hereafter) and the effective reproductive effort (ERE) within and among our colonies, in line of previous studies (see Baillon et al. 2014, 2015; Servetto and Sahade 2016). These data must be considered with caution when compared with those of the other species presented in Table 3 with a known reproductive cycle. In general, those morphologies with polyp leaves present the highest values in reproductive efforts.
Oocyte Diameters
As do other octocorals, sea pens show, within the same autozooid, different oocyte (or spermatocyst) diameters that would correspond to different maturity stages (Chia and Crawford 1973; Servetto and Sahade 2016). However, previous studies on the reproductive cycle of pennatulaceans with polyp leaves, such as Ptilosarcus, Pteroeides (including our present study) and Pennatula, reported that it requires a huge and often unsuccessful effort to try to separate the autozooids individually to assess their sexual products (see the autozooid arrangement within polyp leaves, Fig. 2C, D) (Chia and Crawford 1973; Duncan 1998; Edwards and Moore 2008).
Levitan (1993, 1996) argued that large oocytes would be an easier target for sperm, increasing the probability of fecundation. This also appears to be favored by releasing sperm as cysts into the water column (Eckelbarger et al. 1998). Maximum oocyte diameter in sea pens ranged between 300 µm in Virgularia juncea and 1,200 µm in Anthoptilum murrayi (see Table 3). In our study, and assuming that the largest diameter of spermatocyst and oocyte of our colonies is the largest diameter at the sampling time, the largest oocyte diameter observed was 687 µm, slightly larger than those registered by Duncan (1998) for Pteroeides sp. (550 µm). As occurred in previous studies (e.g. Mladenov and Brady 1987; McClary and Mladenov 1989), Duncan (1998) shown as different oocyte sizes found within polyps of Pteroeides sp. corresponded to different stages of development and were classified into five stages of maturity: stage I (post-spawning, a few remnant large mature oocytes, < 250 μm); stage II (early growth, ~ 100 μm); stage III (previtellogenic oocytes, 100–200 μm; and vitellogenic oocytes, > 200 μm); stage IV-V (mature oocytes, 300 to 520 μm; and ready to spawn, > 300 μm) (Duncan 1998: Fig. 5.2). Then, these oocyte stages of development can be summarized in immature or remnant stage (< 250 μm), and mature stage (> 250 μm) and they are consistent with our oocyte size classes (small oocytes < 250 μm, and large oocytes > 250 μm). However, to validate this assumption the complete cycle of reproduction in Pt. spinosum must be known. This relationship between the size of gametes and their stage of development has also been documented in other sea pens (Servetto and Sahade 2016; Couillard et al. 2021).
Considering that oocyte developmental times of Pteroeides spinosum have been still unknown, similar types of large oocyte diameter has been related to long developmental periods (Orejas et al. 2002). Large oocytes also appear to be related to longer survival of larvae, providing a high nutritional content essential for lecithotrophic larvae (Chia and Crawford 1973). Although it has not been directly observed, oocyte sizes in Pt. spinosum are large enough to facilitate lecithotrophic development, as was suggested for Ptilosarcus guerneyi (687 µm) and Kophobelemnon stelliferum (800 µm) (Chia and Crawford 1973; Rice et al. 1992). Larvae were never observed in the gastrovascular cavities of polyps of our Pt. spinosum colonies. After determining the diameter of the leading cohort of oocytes (LCO) in all Pt. spinosum individual colonies (see Figs. 6 and 7), the size distribution of these LCO did not discriminate between colonies at different stages of maturity, all female colonies had LCO > 400 µm, thus not following the LCO:colony height relationship presented in Pennatula aculeata colonies (Couillard et al. 2021). Perhaps further captures that considerably increase the colonies to examine may offer additional information in this case.
Intracolonial Variation
In octocorals, intracolonial variation in reproductive features has been largely documented as a differential contribution by polyps according to their location within the colony (Brazeau and Lasker 1990; Soong and Lang 1992; Coma et al. 1995a, b; Brito et al. 1997; Orejas et al. 2002; among others).
Some studies in pennatulaceans determined a lineal increase in reproductive contribution of the polyps located from proximal to upper zones (medial and distal), the former commonly appearing empty of reproductive products (e.g. Funiculina quadrangularis in Edwards and Moore 2009; Anthoptilum grandiflorum in Baillon et al. 2014; Halipteris finmarchica in Baillon et al. 2015; Malacobelemnon daytoni in Servetto et al. 2013, Servetto and Sahade 2016; Pennatula aculeata in Couillard et al. 2021). However, the highest estimated mean fecundity in Virgularia juncea was found at the medial zone (Edwards and Moore 2009), as occurred with our colonies of Pteroeides spinosum, in which features such as polyp leaf lengths, mean polyps per leaf and mean oocytes per leaf, reached the highest values at the medial zone of rachis, decreasing towards to both extremes. Some Pt. spinosum male colonies showed slight differences between medial and distal zones, with a similar contribution. In this case, the lower number of spermatocysts per polyp leaf was compensated by a higher number and more congested polyp leaves at the distal portion of the rachis. On the one hand, the higher reproductive contribution in medial-distal zones may be related to the higher number and size of autozooids and polyp leaves in these zones, in comparison to the shorter polyp-leaves often bearing less developed autozooids, as previously suggested for other pennatulacean species. On the other hand, the access to nutritional resources falling from the upper water layers by those autozooids located in the medial-distal zone can provide more matter and energy than that obtained, in quality and amount of particles, by autozooids located in the lower polyp-leaves (Edwards and Moore 2008; Baillon et al. 2014).
Oocyte Diameter Frequency Distribution
It is commonly accepted in octocorals that a frequency-distribution in two clearly defined oocyte size groups represents the overlapping of two cohorts: the first cohort gathering immature oocytes, and kept practically throughout the year; the second cohort, gathering a less numerous oocyte pool at different degrees of maturity, formed in the previous year and that will be released in that season, often over one night (Orejas et al. 2007; Simpson 2009). As an alternative the possible occurrence of a trimodal size distribution of oocytes has been discussed for some pennatulacean species (see Edwards and Moore 2008; Couillard et al. 2021). In the reproductive cycle of Pteroeides sp. mature oocytes were only present from February to April (corresponding to summer and autumn), with very few remnant unspawned oocytes observed in May (late autumn) (Duncan 1998). This means that between late summer and autumn nearly all vitellogenic oocytes became mature and were spawned. In parallel, Duncan observed a cohort of immature oocytes in all captures made over the two years. Although, as seen before, the developmental periods of oocytes in Pteroeides spinosum are still unknown, our results based on colonies collected in early summer and with a high percentage of large oocytes, follow the developmental line observed in Pteroeides sp. by Duncan (1998) (see below). The oocyte diameter frequency distribution for each individual colony not revealed the potential presence of a third modal ‘peak’ (as occurred for example in Pennatula aculeata, see Edwards and Moore 2008, Fig. 2) but rather the bimodal distribution was constant in all colonies examined, as occurred in Pteroeides sp. (Duncan 1998).
Fecundity
In most sessile invertebrate marine organisms, fecundity is often determined indirectly from counts of oocytes present, with the assumption that all or most become viable offspring (Brazeau and Lasker 1989; Benayahu and Loya 1983; Langton et al. 1990). In agreement with other authors (e.g. Duncan 1998), we consider here the term reproductive effort to be more appropriate, because it was not possible to assess the actual number of larvae produced. The mean potential relative fecundity (PRF) of our Pteroeides spinosum colonies (ranged between 7 and 17 oocytes per polyp) showed a similar range to other pennatulaceans, such as Renilla koellikeri (7–8 oocytes per polyp, Tremblay et al. 2004).
Quantifying different oocyte maturity stages within the colony offers more reliable information about the reproductive stage of the colony and its reproductive effort (Servetto and Sahade 2016). However, comparisons with previous reproductive studies must be made with caution because some data are based on potential reproductive effort (PRE) (e.g. Tremblay et al. 2004; Soong 2005; Pires et al. 2009) while others did not include immature oocytes in their fecundity estimates (ERE) (e.g. Chia and Crawford 1973; Tyler et al. 1995; Baillon et al. 2014; Hamel et al. 2020) (see Table 3). Moreover, some of these species have the complete reproductive cycle examined while others are based on scarce samples or punctual samplings (Chia and Crawford 1973; Eckelbarger et al. 1998). Our colonies of Pteroeides spinosum showed ERE values similar to the ERE of Pteroeides sp. (Duncan 1998). However, without a seasonal sampling program, inferences on colonial maturity stages and spawning events in Pt. spinosum must remain speculative. Future samplings of this population in different seasons might offer more information about the maturation cycle and verify spawning events with more accuracy.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Abdelsalam KM (2014) Faunistic study of benthic Pennatulacea (Cnidaria, Octocorallia) from the Northern coast of Egypt. Egypt J Aquat Res 40(3):261–268
Baillon S, Hamel JF, Wareham VE, Mercier A (2012) Deep cold-water corals as nurseries for fish larvae. Front Ecol Environ 10(7):351–356
Baillon S, Hamel JF, Wareham VE, Mercier A (2014) Seasonality in reproduction of the deep-water pennatulacean coral Anthoptilum grandiflorum. Mar Biol 161(1):29–43
Baillon S, Hamel JF, Mercier A (2015) Protracted oogenesis and annual reproductive periodicity in the deep-sea pennatulacean Halipteris finmarchica (Anthozoa, Octocorallia). Mar Ecol 36(4):1364–1378
Bayer FM, Grasshoff M, Verseveldt J (1983) Illustrated trilingual glossary of morphological and anatomical terms applied to Octocorallia. EJ Brill/ Dr. Backhuys, Leiden, pp 75
Benayahu Y, Loya Y (1983) Surface brooding in the Red Sea soft coral Parerythropodium fulvum fulvum (Forskal, 1775). Biol Bull 165(2):353–369
Bohadsch JB (1761) De quibusdam animalibus marinis, eorumque proprietabibus, orbi litterario vel nondum vel minus notis, liber cum nonnullis tabulis aeri incisis, ab auctore super vivis animalibus delineatis. Dresdae: apud G.C. Walther
Brazeau DA, Lasker HR (1989) The reproductive cycle and spawning in a Caribbean gorgonian. Biol Bull 176(1):1–7
Brazeau DA, Lasker HR (1990) Sexual reproduction and external brooding by the Caribbean gorgonian Briareum asbestinum. Mar Biol 104(3):465–474
Brazeau DA, Lasker HR (1992) Reproductive success in the Caribbean octocoral Briareum asbestinum. Mar Biol 114(1):157–163
Brito TA, Tyler PA, Clarke A (1997) Reproductive biology of the Antarctic octocoral Thouarella variabilis Wright and Studer 1889. In: Hartog JC den (ed) Proc 6th Int Conf on coelenterate biology, Leiden, pp 63–69
Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG et al (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol 31:21–50
Carney RS (2005) In: Gibson RN, Oceanography and marine biology: An annual review 43:211–278
Chia FS, Crawford BJ (1973) Some observations on gametogenesis, larval development and substratum selection of the sea pen Ptilosarcus guerneyi. Mar Biol 23:73–82
Chimienti G, Angeletti L, Mastrototaro F (2018) Withdrawal behaviour of the red sea pen Pennatula rubra (Cnidaria: Pennatulacea). Eur Zool J 85(1):64–70
Clippele LH, Buhl-Mortensen P, Buhl-Mortensen L (2015) Fauna associated with cold water gorgonians and sea pens. Cont Shelf Res 105:67–78
Coelho MA, Lasker HR (2014) Reproductive biology of the Caribbean brooding octocoral Antillogorgia hystrix. Invertebr Biol 133(4):299–313
Coffroth MA, Lasker HR (1998) Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution 52(2):379–393
Coma R, Ribes M, Zabala M, Gili JM (1995a) Reproduction and cycle of gonadal development in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117:173–183
Coma R, Ribes M, Zabala M, Gili JM (1995b) Sexual reproductive effort in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117(1):185–192
Connor DW, Allen JH, Golding NEIL, Lieberknecht LM, Northen KO et al (2003) The national marine habitat classification for Britain and Ireland. JNCC Peterborough
Couillard CM, Sainte-Marie B, Dionne H (2021) Late maturity and evidence for female biennial spawning in the sea pen Pennatula aculeata (Anthozoa, Pennatulacea) in eastern Canada. Invertebr Biol 140(4):e12351
Curd A (2010) Background document for sea pen and burrowing megafauna communities. Publication 481(2010):26
Davies JS, Guillaumont B, Tempera F, Vertino A, Beuck L et al (2017) A new classification scheme of European cold-water coral habitats: implications for ecosystem-based management of the deep sea. Deep Sea Res Part II Top Stud Oceanogr 145:102–109
Duncan JC (1998) Biology of the sea pen Pteroeides sp. in Fiordland, New Zealand. PhD dissertation. University of Otago, New Zealand, pp 88
Eckelbarger KJ, Tyler PA, Langton RW (1998) Gonadal morphology and gametogenesis in the sea pen Pennatula aculeata (Anthozoa: Pennatulacea) from the Gulf of Maine. Mar Biol 132:677–690
Edwards DCB, Moore CG (2008) Reproduction in the sea pen Pennatula phosphorea (Anthozoa: Pennatulacea) from the west coast of Scotland. Mar Biol 155:303–314
Edwards DCB, Moore CG (2009) Reproduction in the sea pen Funiculina quadrangularis (Anthozoa: Pennatulacea) from the west coast of Scotland. Estuar Coast Shelf Sci 82:161–168
Ellis J (1764) An account of the sea pen, or Pennatula phosphorea of Linnaeus; likewise a description of a new species of sea pen, found on the coast of South-Carolina, with observations on sea-pens in general. In a letter to the honourable Coote Molesworth, Esq; M.D. and F.R.S. from John Ellis, esq; F.R.S. and member of the Royal Academy of Upsal. Philos Trans R Soc Lond B 53:419–435
Ellis J, Solander D (1786) The natural history of many curious and uncommon zoophytes, collected from various parts of the globe by the late John Ellis systematically arranged and described by the late Daniel Solander. London, printed for Benjamin White and Son, at Horace’s Head, Fleet-Street; and Peter Elmsly, in the Strand. 206 pp
Fiorillo I, Rossi S, Alvà V, Gili JM, López-González PJ (2013) Seasonal cycle of sexual reproduction of the Mediterranean soft coral Alcyonium acaule (Anthozoa, Octocorallia). Mar Biol 160(3):719–728
Flint HC, Waller RG, Tyler PA (2007) Reproductive ecology of Fungiacyathus marenzelleri from 4100 m depth in the northeast Pacific Ocean. Mar Biol 151(3):843–849
García-Cárdenas FJ, López-González PJ (2022) Biogeographic patterns in sea pens (Octocorallia: Pennatulacea). Mar Biodivers. Accepted MS.
Gili JM, Pagès F (1987) Pennatuláceos (Cnidaria, Anthozoa) recolectados en la plataforma continental catalana (Mediterraneo occidental). Misc Zool 11:25–39
Gray JE (1870) Catalogue of sea-pens or Pennatulariidae in the collection of the British Museum. British Museum, London, p 40
Hamel JF, Wareham-Hayes VE, Mercier A (2020) Reproduction of a bathyal pennatulacean coral in the Canadian Arctic. Deep-Sea Res I 162:103321
Herklots JA (1858) Notices pour servir à l’étude des polypiers nageurs ou pennatulidés. Bijdr Dierkd 7:1–31
International Commission on Zoological Nomenclature (ICZN) (1944) Opinion 185. Suppression of Bohadsch (J.B.), De quibusdam Animalibus marinis, 1761, and of the German translation thereof published by Leske (N.G.) in 1776). Opinions and Declarations Rendered by the International Commission on Zoological Nomenclature 3 (Part 4):37–52
Jones LA, Hiscock K, Connor DW (2000) Marine habitat reviews, a summary of ecological requirements and sensitivity characteristics for the conservation and management of Marine SAC’s. UK Marine SAC’s Project Report. Peterborough: Joint Nature Conservation Committee, pp 178
Kükenthal W (1915) Pennatularia. Das Tierreich. Verlag von R. Friedländer Und Sohn, Berlin 43:1–132
Langton RW, Langton EW, Theroux RB, Uzmann JR (1990) Distribution, behaviour and abundance of sea pens, Pennatula aculeata, in the Gulf of Maine. Mar Biol 107:463–469
Laverick JH, Andradi-Brown DA, Rogers AD (2017) Using light-dependent scleractinia to define the upper boundary of mesophotic coral ecosystems on the reefs of Utila. Honduras Plos One 12(8):e0183075
Levitan DR (1993) The importance of sperm limitation to the evolution of egg size in marine invertebrates. Am Nat 141:517–536
Levitan DR (1996) Predicting optimal and unique egg sizes in free-spawning marine invertebrates. Am Nat 148:174–188
Lopes VM, Baptista M, Pimentel MS, Repolho T, Narciso L et al (2012) Reproduction in Octocorallia: synchronous spawning and asynchronous oogenesis in the pennatulid Veretillum cynomorium. Mar Biol Res 8(9):893–900
Marshall AM, Fowler GH (1887) Report on the Pennatulida dredged by H. M. S. ‘Porcupine.’ Trans Roy Soc Edinburgh 33(2):453–464
McClary DJ, Mladenov PV (1989) Reproductive pattern in the brooding and broadcasting sea star Pteraster militaris. Mar Biol 103(4):531–540
Mladenov PV, Brady K (1987) Reproductive cycle of the Caribbean feather star Nemaster rubiginosa (Echinodermata: Crinoidea). Mar Ecol 8(4):313–325
Nurdin N, Lanuru M, Akbar AS, Kartika I, Komatsu T (2019) Accuracy of unsupervised classification to determine coral health using SPOT-6 and Sentinel-2A. Int Arch Photogramm Remote Sens Spat Inf Sci Vol XLII-4/W16 6th Int Conf on Geomatics and Geospatial Technology
Ocaña Ó, Wirtz P (2007) New records of Pennatulacea (Cnidaria, Octocorallia) from Madeira island. Arquipélago. Life Mar Sci 24:49–51
Orejas C, López-González PJ, Gili JM, Teixidó N (2002) Distribution and reproductive ecology of the Antarctic octocoral Ainigmaptilon antarcticum in the Weddell Sea. Mar Ecol Prog Ser 231:101–114
Orejas C, Gili JM, López-González PJ, Hasemann C, Arntz WE (2007) Reproduction patterns of four Antarctic octocorals in the Weddell Sea: an inter-specific, shape, and latitudinal comparison. Mar Biol 150:551–563
Page CA, Lasker HR (2012) Effects of tissue loss, age and size on fecundity in the octocoral Pseudopterogorgia elisabethae. J Exp Mar Biol Ecol 434–435:47–52
Pax FA, Müller I (1953) Die Anthozoen fauna von der Bucht von Kastela bei Split. Acta Adriat 5(1):14–35
Pax FA, Müller I (1955) Die Korallentiere der Adria. Die Aquarien- und Terrarien Zeitschrift, 8,10–12; 39–40; 67–69
Pax FA, Müller I (1959) The role of the Wroclaw Zoological Institute and Museum in the study of the fauna of Anthozoa in the Adriatic Sea. Przeglad Zoologiczny 3(1):44–53
Pires DO, Castro CB, Silva JC (2009) Reproductive biology of the deep-sea pennatulacean Anthoptilum murrayi (Cnidaria, Octocorallia). Mar Ecol Prog Ser 397:103–112
Porporato EMD, Mangano MC, De Domenico F, Giacobbe S, Spanò N (2014) First observation of Pteroeides spinosum (Anthozoa: Octocorallia) fields in a Sicilian coastal zone (Central Mediterranean Sea). Mar Biodivers 44:589–592
Quintanilla E, Gili JM, López-González PJ, Tsounis G, Madurell T et al (2013) Sexual reproductive cycle of the epibiotic soft coral Alcyonium coralloides (Octocorallia, Alcyonacea). Aquat Biol 18(2):113–124
Rice AL, Tyler PA, Paterson GJL (1992) The pennatulid Kophobelemnon stelliferum (Cnidaria: Octocorallia) in the porcupine seabight (north-east Atlantic Ocean). J Mar Biolog Assoc UK 72:417–434
Roberts CM (2002) Deep impact: the rising toll of fishing in the deep sea. Trends Ecol Evol 17:242–245
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312(5773):543–547
Rossi L (1971) Guida a cnidari e ctenofori della fauna italiana. Quaderni Sta Idrobiol Milano 2:1–101
Servetto N, Sahade R (2016) Reproductive seasonality of the Antarctic sea pen Malacobelemnon daytoni (Octocorallia, Pennatulacea, Kophobelemnidae). PLoS ONE 11(10):e0163152
Servetto N, Torre L, Sahade R (2013) Reproductive biology of the Antarctic “sea pen” Malacobelemnon daytoni (Octocorallia, Pennatulacea, Kophobelemnidae). Polar Res 32(1):20040
Simpson A (2009) Reproduction in octocorals (Subclass Octocorallia): a review of published literature. http://www.ucs.louisiana.edu/~scf4101/Bambooweb
Soong K (2005) Reproduction and colony integration of the sea pen Virgularia juncea. Mar Biol 146:1103–1109
Soong K, Lang JC (1992) Reproductive integration in reef corals. Biol Bull Rev 183(3):418–431
Theodor J (1967) Contribution a l’étude des gorgones VII. Ecologie et comportement de la planula. Vie Milieu 18:291–301
Topçu EN, Öztürk B (2015) Composition and abundance of octocorals in the Sea of Marmara, where the Mediterranean meets the Black Sea. Sci Mar 79(1):125–135
Tremblay ME, Henry J, Anctil M (2004) Spawning and gamete follicle rupture in the cnidarian Renilla koellikeri: effects of putative neurohormones. Gen Comp Endocrinol 137:9–18
Tyler PA, Bronsdon SK, Young CM, Rice AL (1995) Ecology and gametogenic biology of the genus Umbellula (Pennatulacea) in the North Atlantic Ocean. Int Rev Hydrobiol 80(2):187–199
Weinberg S, Weinberg F (1979) The life cycle of a gorgonian: Eunicella singularis (Esper, 1794). Bijdr Dierkd 48:127–140
Williams GC (1995) Living genera of sea pens (Coelenterata: Octocorallia: Pennatulacea): illustrated key and synopses. Zool J Linn Soc 113:93–140
Williams GC (2011) The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea). PLoS ONE 6(7):e22747
Zar JH (1984) Biostatistical analysis. 2nd. Prentice Hall, USA
Acknowledgements
The authors would like to thank the scientists and crew on board the R/V García del Cid during the "INDEMARES-CSIC VI", cruise to Cap de Creus, as well as many colleagues on board, without whose help it would not have been possible to collect the specimens for this work. Special thanks are addressed to Josep-Maria Gili for his continuous support to benthic research in the NW Mediterranean Sea. This research was funded by LIFE+ INDEMARES project (LIFE07/NAT/E/000732). The authors are also grateful for the criticisms and suggestions provided by three anonymous referees and the editorial board of Thalassas. Mr. Tony Krupa is thanked for reviewing the English version.
Funding
Funding for open access publishing: Universidad de Sevilla/CBUA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Cárdenas, F.J., López-González, P.J. Some Observations on the Reproductive Biology of the Mediterranean Pennatulacean Pteroeides Spinosum (Ellis and Solander, 1786) (Cnidaria: Octocorallia: Pennatulacea). Thalassas 39, 181–197 (2023). https://doi.org/10.1007/s41208-022-00505-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-022-00505-6